
Contributions
Abstract: S228
Type: Oral Presentation
Session title: Lymphoma - Translational research
Background
The Non-SMC Condensin II Complex Subunit D3 (NCAPD3) is a key component of condensin. Existing evidence confirms that somatic mutations in NCAPD3 are linked to deregulated pathways and a large number of diseases, including cancers. However, little is known regarding its biological and clinical significance in diffuse large B-cell lymphoma (DLBCL).
Aims
This series of experiments are aimed to explore the pattern of expression, functional mechanisms and potential clinical relevance of NCAPD3 in DLBCL.
Methods
Archived, paraffin-embedded samples extracted from 70 de novo DLBCL patients and 30 reactive hyperplasia cases were collected with informed consents. The GEO and TCGA database were obtained to aid in subsequent survival analysis. Empty lentiviral vectors (shControl) or those coding for shNCAPD3 were stably transfected into DLBCL cell lines which were then assessed using RNA-sequencing (RNA-seq) analysis. DLBCL xenograft models were established by injecting mice subcutaneously with DLBCL cells. ARRIE guidelines were strictly adhered to for all experiments.
Results
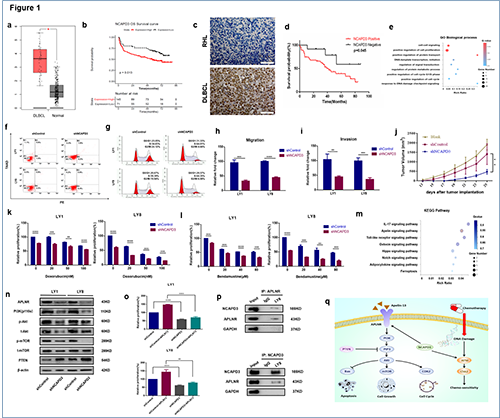
TCGA database demonstrated that the expression level of NCAPD3 in DLBCL was raised in comparison to those of normal tissues (Fig. 1a). Kaplan-Meier survival curve revealed that higher NCAPD3 expression levels in DLBCL cases were linked to shorter overall survival times according to GEO database (Fig. 1b). IHC staining showed higher expression levels of NCAPD3 in DLBCL tissues than those in control tissues and significantly related to poorer prognosis (Table 1, Fig 1c, d). High expression levels of NCAPD3 mRNA and protein were confirmed in DLBCL cell lines as well.
Functional enrichment analyses of NCAPD3 according to RNA-seq revealed that NCAPD3 was correlated strongly to cell proliferation, cell cycle and DNA damage signaling (Fig 1e). Knockdown of NCAPD3 significantly suppressed cell proliferation, promoted cell apoptosis, induced cell cycle arrest and impaired cell migration and invasion (Fig 1f-i). Tumors with NCAPD3-repression displayed suppressed tumor growth (Fig 1j) and diminished levels of Ki-67 in vivo. Also, NCAPD3 depletion in DLBCL were proved to enhance the chemo-sensitivity of DLBCL through the DNA damage signaling (Fig 1k, l).
With KEGG results derived from the RNA-seq data, NCAPD3 was found to be functionally enriched in the Apelin signaling pathway (Fig. 1m). NCAPD3 and APLNR revealed a significant association in DLBCL according to TCGA database. Western blot demonstrated a decrease in the levels of APLNR and its downstream PI3K/Akt/mTOR components in shNCAPD3 cells in contrast to shControl cells (Fig 1n). The APLNR activator APLN-13 were exposed to DLBCL cells transfected with either shNCAPD3 or shControl, cells containing shNCAPD3 demonstrated decreased proliferation induction (Fig. 1o). The co-IP experiment suggested that NCAPD3 regulates the Apelin/PI3K pathway by directly binding to APLNR (Fig. 1p). In summary, NCAPD3 may modulate DLBCL progression through regulation of the Apelin/PI3K and DNA damage response signaling pathway (Fig. 1q).
Conclusion
Our clinical and fundamental results highlight the pivotal oncogenic role of NCAPD3 in DLBCL for the first time, suggesting blockade of NCAPD3 as a novel therapeutic approach in DLBCL mediating through the Apelin/PI3K signaling pathway.
Keyword(s): Diffuse large B cell lymphoma, Oncogene, Targeted therapy
Abstract: S228
Type: Oral Presentation
Session title: Lymphoma - Translational research
Background
The Non-SMC Condensin II Complex Subunit D3 (NCAPD3) is a key component of condensin. Existing evidence confirms that somatic mutations in NCAPD3 are linked to deregulated pathways and a large number of diseases, including cancers. However, little is known regarding its biological and clinical significance in diffuse large B-cell lymphoma (DLBCL).
Aims
This series of experiments are aimed to explore the pattern of expression, functional mechanisms and potential clinical relevance of NCAPD3 in DLBCL.
Methods
Archived, paraffin-embedded samples extracted from 70 de novo DLBCL patients and 30 reactive hyperplasia cases were collected with informed consents. The GEO and TCGA database were obtained to aid in subsequent survival analysis. Empty lentiviral vectors (shControl) or those coding for shNCAPD3 were stably transfected into DLBCL cell lines which were then assessed using RNA-sequencing (RNA-seq) analysis. DLBCL xenograft models were established by injecting mice subcutaneously with DLBCL cells. ARRIE guidelines were strictly adhered to for all experiments.
Results
TCGA database demonstrated that the expression level of NCAPD3 in DLBCL was raised in comparison to those of normal tissues (Fig. 1a). Kaplan-Meier survival curve revealed that higher NCAPD3 expression levels in DLBCL cases were linked to shorter overall survival times according to GEO database (Fig. 1b). IHC staining showed higher expression levels of NCAPD3 in DLBCL tissues than those in control tissues and significantly related to poorer prognosis (Table 1, Fig 1c, d). High expression levels of NCAPD3 mRNA and protein were confirmed in DLBCL cell lines as well.
Functional enrichment analyses of NCAPD3 according to RNA-seq revealed that NCAPD3 was correlated strongly to cell proliferation, cell cycle and DNA damage signaling (Fig 1e). Knockdown of NCAPD3 significantly suppressed cell proliferation, promoted cell apoptosis, induced cell cycle arrest and impaired cell migration and invasion (Fig 1f-i). Tumors with NCAPD3-repression displayed suppressed tumor growth (Fig 1j) and diminished levels of Ki-67 in vivo. Also, NCAPD3 depletion in DLBCL were proved to enhance the chemo-sensitivity of DLBCL through the DNA damage signaling (Fig 1k, l).
With KEGG results derived from the RNA-seq data, NCAPD3 was found to be functionally enriched in the Apelin signaling pathway (Fig. 1m). NCAPD3 and APLNR revealed a significant association in DLBCL according to TCGA database. Western blot demonstrated a decrease in the levels of APLNR and its downstream PI3K/Akt/mTOR components in shNCAPD3 cells in contrast to shControl cells (Fig 1n). The APLNR activator APLN-13 were exposed to DLBCL cells transfected with either shNCAPD3 or shControl, cells containing shNCAPD3 demonstrated decreased proliferation induction (Fig. 1o). The co-IP experiment suggested that NCAPD3 regulates the Apelin/PI3K pathway by directly binding to APLNR (Fig. 1p). In summary, NCAPD3 may modulate DLBCL progression through regulation of the Apelin/PI3K and DNA damage response signaling pathway (Fig. 1q).

Conclusion
Our clinical and fundamental results highlight the pivotal oncogenic role of NCAPD3 in DLBCL for the first time, suggesting blockade of NCAPD3 as a novel therapeutic approach in DLBCL mediating through the Apelin/PI3K signaling pathway.
Keyword(s): Diffuse large B cell lymphoma, Oncogene, Targeted therapy


