
Contributions
Abstract: S227
Type: Oral Presentation
Session title: Lymphoma - Translational research
Background
Clonal hematopoiesis (CH) is frequent in the elderly and predisposes to blood tumors, mostly of myeloid and T-cell origin. CH incidence and trajectories are not well characterized in classical Hodgkin lymphoma (cHL), a B-cell neoplasm frequent in the young and uniquely featuring rare cancer cells embedded in a supportive microenvironment largely of hematopoietic origin.
Aims
To assess whether cHL belongs to the spectrum of hematologic malignancies associated with CH, here we report CH frequency and tissue distribution in 40 cHL patients, including the surprising findings on a young case with massive CH who developed acute myeloid leukemia (AML) following therapy for cHL.
Methods
We subjected 40 cHL cases to whole-exome and/or targeted sequencing (mean unique coverage ~150X and ~1000X, respectively) of tumor cells microdissected from the tissue biopsy in parallel with matched non-neoplastic blood cells (n=26 cases) or microdissected lymphoid cells (n=14 cases).
Results
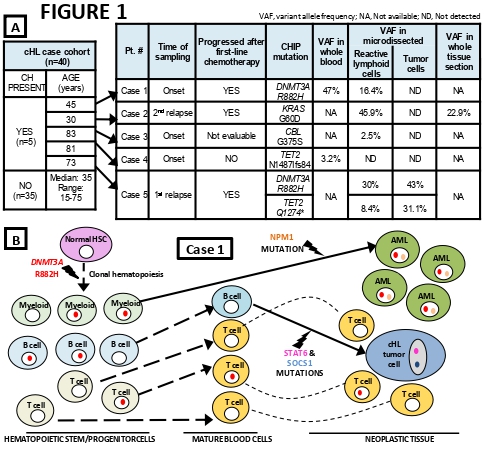
5/40 cases (12.5%) had blood and/or tissue CH, including 3/5 with >70 years and 2/35 <70 years (Fig. 1A). In 3/5 cases with CH (aged 30, 45 and 73 years), CH extensively spread through the non-neoplastic tissue microenvironment (92%, 32% and 60% of cells), being respectively driven by mutant KRASG60D, DNMT3AR882H, and DNMT3AR882H+TET2Q1274* (Fig. 1A). Notably, in the latter case (Case 5) CH originated also the tumor cell clone, which was infected by the Epstein-Barr virus and had almost no other somatic mutations exome-wide; this is the first description, in a human B-cell lymphoma clone, of mutant DNMT3AR882, a hotspot in myeloid and T-cell neoplasms.
In the other case with DNMT3A-mutant CH (Case 1; Fig.1B), the same variant R882H caused a massive CH (94% of blood leukocytes; 96% of blood B cells) that was surprisingly absent from the tumor cell clone (instead carrying STAT6 and SOCS1 mutations, typical of cHL) and led, 6 years after therapy for cHL, to a likely therapy-unrelated NPM1-mutated acute myeloid leukemia (with normal karyotype, wild-type TP53 and PPM1D). Thus, CH trajectories must be fully disentangled to correctly interpret the histogenesis and pathogenesis of multiple myeloid and lymphoid neoplasms arising in patients with CH, as the latter (even when massive) does not necessarily represent the shared genetic background of all neoplastic clones.
Finally, in the 3 cases with extensive CH propagation in the cHL tissue microenvironment (mostly represented by T cells), we observed a polyclonal status of T-cell receptor gene rearrangements, confirming that the mutations underlying CH had occurred in a bone marrow stem or progenitor cell before T-cell lineage commitment. Interestingly, all these 3 cases progressed after first-line chemotherapy, versus 11/35 (31%; p-value 0.043) evaluable cases with absent or non-extensive tissue CH, who otherwise had similar clinical features and received the same first-line therapy (not shown).
Conclusion
CH can be observed in the cHL tissue, can originate the tumor clone and can propagate to large part of its microenvironment. Considering that both tumor cells and the microenvironment supporting its growth play key roles in cHL pathogenesis, CH might contribute to the development of this lymphoma and influence its prognosis.
Keyword(s): Acute myeloid leukemia, Clonal expansion, Hodgkin's lymphoma, Microenvironment
Abstract: S227
Type: Oral Presentation
Session title: Lymphoma - Translational research
Background
Clonal hematopoiesis (CH) is frequent in the elderly and predisposes to blood tumors, mostly of myeloid and T-cell origin. CH incidence and trajectories are not well characterized in classical Hodgkin lymphoma (cHL), a B-cell neoplasm frequent in the young and uniquely featuring rare cancer cells embedded in a supportive microenvironment largely of hematopoietic origin.
Aims
To assess whether cHL belongs to the spectrum of hematologic malignancies associated with CH, here we report CH frequency and tissue distribution in 40 cHL patients, including the surprising findings on a young case with massive CH who developed acute myeloid leukemia (AML) following therapy for cHL.
Methods
We subjected 40 cHL cases to whole-exome and/or targeted sequencing (mean unique coverage ~150X and ~1000X, respectively) of tumor cells microdissected from the tissue biopsy in parallel with matched non-neoplastic blood cells (n=26 cases) or microdissected lymphoid cells (n=14 cases).
Results
5/40 cases (12.5%) had blood and/or tissue CH, including 3/5 with >70 years and 2/35 <70 years (Fig. 1A). In 3/5 cases with CH (aged 30, 45 and 73 years), CH extensively spread through the non-neoplastic tissue microenvironment (92%, 32% and 60% of cells), being respectively driven by mutant KRASG60D, DNMT3AR882H, and DNMT3AR882H+TET2Q1274* (Fig. 1A). Notably, in the latter case (Case 5) CH originated also the tumor cell clone, which was infected by the Epstein-Barr virus and had almost no other somatic mutations exome-wide; this is the first description, in a human B-cell lymphoma clone, of mutant DNMT3AR882, a hotspot in myeloid and T-cell neoplasms.
In the other case with DNMT3A-mutant CH (Case 1; Fig.1B), the same variant R882H caused a massive CH (94% of blood leukocytes; 96% of blood B cells) that was surprisingly absent from the tumor cell clone (instead carrying STAT6 and SOCS1 mutations, typical of cHL) and led, 6 years after therapy for cHL, to a likely therapy-unrelated NPM1-mutated acute myeloid leukemia (with normal karyotype, wild-type TP53 and PPM1D). Thus, CH trajectories must be fully disentangled to correctly interpret the histogenesis and pathogenesis of multiple myeloid and lymphoid neoplasms arising in patients with CH, as the latter (even when massive) does not necessarily represent the shared genetic background of all neoplastic clones.
Finally, in the 3 cases with extensive CH propagation in the cHL tissue microenvironment (mostly represented by T cells), we observed a polyclonal status of T-cell receptor gene rearrangements, confirming that the mutations underlying CH had occurred in a bone marrow stem or progenitor cell before T-cell lineage commitment. Interestingly, all these 3 cases progressed after first-line chemotherapy, versus 11/35 (31%; p-value 0.043) evaluable cases with absent or non-extensive tissue CH, who otherwise had similar clinical features and received the same first-line therapy (not shown).

Conclusion
CH can be observed in the cHL tissue, can originate the tumor clone and can propagate to large part of its microenvironment. Considering that both tumor cells and the microenvironment supporting its growth play key roles in cHL pathogenesis, CH might contribute to the development of this lymphoma and influence its prognosis.
Keyword(s): Acute myeloid leukemia, Clonal expansion, Hodgkin's lymphoma, Microenvironment


