
Contributions
Abstract: S206
Type: Oral Presentation
Session title: Hodgkin lymphoma - Clinical
Background
Camrelizumab (a humanized high-affinity IgG4 mAb against PD-1) showed potent antitumor activity, well tolerance, and controllable safety in Chinese patients with relapsed or refractory classical Hodgkin lymphoma (r/r cHL), based on the primary analysis results of a multicenter, phase 2 study (ClinicalTrials.gov, NCT03155425). The long-term efficacy and safety of camrelizumab are warranted to be assessed.
Aims
To report the extended follow-up data regarding the durability of response, survival benefit, and long-term medication safety of camrelizumab in r/r cHL patients.
Methods
Between Jun 9, 2017 and Sep 18, 2017, 75 patients who had failed to achieve a remission or experienced progression after autologous stem cell transplantation or had received at least two lines of systemic chemotherapies were enrolled in this study. Camrelizumab at a dose of 200 mg were administrated every two weeks. The primary endpoint was objective response rate per independent review committee (IRC).
Results
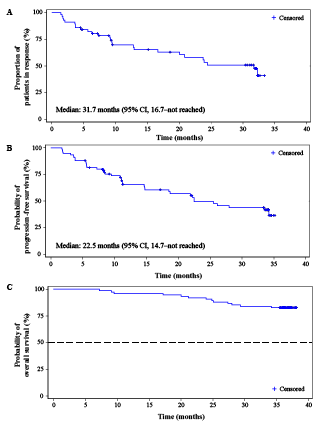
As of Aug 31, 2020, the median follow-up duration was 36.2 months (range, 7.2–38.1). Per IRC assessment, objective responses were achieved in 57 patients (76.0%; 95% CI, 64.7–85.1), similar to result of the primary analysis. The Kaplan-Meier estimated median duration of response was 31.7 months (95% CI, 16.7–not reached; Figure A). At data cutoff, 50.7% of patients (38/75) had disease progression or died. The median progression-free survival was 22.5 months (95% CI, 14.7–not reached; Figure B). Thirteen (17.3%) deaths occurred, and the median overall survival was not reached (Figure C). The 36-month OS rate was 82.7% (95% CI, 72.0–89.5). The most common treatment-related adverse events (TRAEs) included reactive capillary endothelial proliferation (RCEP; 73/75, 97.3%), pyrexia (33/75, 44.0%), upper respiratory tract infection (27/75, 36.0%), and hypothyroidism (24/75, 32.0%). Despite a high incidence, all RCEP were grade 1 (59/73, 80.8%) or grade 2 (14/73, 19.2%) in severity. In general, RCEP occurred early during treatment; the median time to onset was 0.9 months (range, 0.1–2.5). Re-occurrence of RCEP was very rare (8 out of 42 patients who were still on camrelizumab therapy after 12 months, 19.0%; 2 out of 32 patients who were still on camrelizumab therapy after 24 months, 6.3%). Complete resolution of all RCEP lesions was observed in 49 of the 73 patients (67.1%), and the median time to regression was 8.2 months (range, 1.7–30.8). Grade 3 or 4 TRAEs were reported in 26 (34.7%) patients. No treatment-related deaths occurred. TRAEs led to treatment discontinuation only in 5 (6.7%) patients. No new safety signals were identified.
Conclusion
With extended follow-up, camrelizumab monotherapy continues to provide a robust and durable response, long progression-free and overall survival, as well as manageable safety in patients with r/r cHL.
Keyword(s): Clinical trial, Hodgkin's lymphoma
Abstract: S206
Type: Oral Presentation
Session title: Hodgkin lymphoma - Clinical
Background
Camrelizumab (a humanized high-affinity IgG4 mAb against PD-1) showed potent antitumor activity, well tolerance, and controllable safety in Chinese patients with relapsed or refractory classical Hodgkin lymphoma (r/r cHL), based on the primary analysis results of a multicenter, phase 2 study (ClinicalTrials.gov, NCT03155425). The long-term efficacy and safety of camrelizumab are warranted to be assessed.
Aims
To report the extended follow-up data regarding the durability of response, survival benefit, and long-term medication safety of camrelizumab in r/r cHL patients.
Methods
Between Jun 9, 2017 and Sep 18, 2017, 75 patients who had failed to achieve a remission or experienced progression after autologous stem cell transplantation or had received at least two lines of systemic chemotherapies were enrolled in this study. Camrelizumab at a dose of 200 mg were administrated every two weeks. The primary endpoint was objective response rate per independent review committee (IRC).
Results
As of Aug 31, 2020, the median follow-up duration was 36.2 months (range, 7.2–38.1). Per IRC assessment, objective responses were achieved in 57 patients (76.0%; 95% CI, 64.7–85.1), similar to result of the primary analysis. The Kaplan-Meier estimated median duration of response was 31.7 months (95% CI, 16.7–not reached; Figure A). At data cutoff, 50.7% of patients (38/75) had disease progression or died. The median progression-free survival was 22.5 months (95% CI, 14.7–not reached; Figure B). Thirteen (17.3%) deaths occurred, and the median overall survival was not reached (Figure C). The 36-month OS rate was 82.7% (95% CI, 72.0–89.5). The most common treatment-related adverse events (TRAEs) included reactive capillary endothelial proliferation (RCEP; 73/75, 97.3%), pyrexia (33/75, 44.0%), upper respiratory tract infection (27/75, 36.0%), and hypothyroidism (24/75, 32.0%). Despite a high incidence, all RCEP were grade 1 (59/73, 80.8%) or grade 2 (14/73, 19.2%) in severity. In general, RCEP occurred early during treatment; the median time to onset was 0.9 months (range, 0.1–2.5). Re-occurrence of RCEP was very rare (8 out of 42 patients who were still on camrelizumab therapy after 12 months, 19.0%; 2 out of 32 patients who were still on camrelizumab therapy after 24 months, 6.3%). Complete resolution of all RCEP lesions was observed in 49 of the 73 patients (67.1%), and the median time to regression was 8.2 months (range, 1.7–30.8). Grade 3 or 4 TRAEs were reported in 26 (34.7%) patients. No treatment-related deaths occurred. TRAEs led to treatment discontinuation only in 5 (6.7%) patients. No new safety signals were identified.

Conclusion
With extended follow-up, camrelizumab monotherapy continues to provide a robust and durable response, long progression-free and overall survival, as well as manageable safety in patients with r/r cHL.
Keyword(s): Clinical trial, Hodgkin's lymphoma


