
Contributions
Abstract: S205
Type: Oral Presentation
Session title: Hodgkin lymphoma - Clinical
Background
Historically, nearly all relapses in classical Hodgkin lymphoma (cHL) occur within 5 years of treatment (Radford, BMJ 1997). In the phase 3 ECHELON-1 study (NCT01712490) in patients (pts) with newly-diagnosed Stage III/IV cHL, treatment with brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine (A+AVD) significantly improved modified progression-free survival (PFS) per independent review facility vs doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) (Connors et al, NEJM 2018). After 3 and 4 years’ follow-up, sustained PFS per investigator (INV) benefits were seen with A+AVD vs ABVD in the intent-to-treat (ITT) population as well as across most key pt subgroups (Bartlett, Blood 2019; Straus, Blood 2020). These improvements were irrespective of interim positron emission tomography (PET) scan status, disease stage, and baseline disease risk factor score.
Aims
We report updated efficacy and safety results after 5-years’ follow-up of the ECHELON-1 study.
Methods
Pts with previously untreated Stage III/IV cHL were randomized to receive ≤6 cycles of intravenous A+AVD (n=664) or ABVD (n=670) on days 1 and 15 of a 28-day cycle. After cycle 2, pts had an interim PET scan (PET2). Analyses were performed after extended follow-up (cutoff date Sept’ 18, 2020) to assess PFS per INV, peripheral neuropathy (PN) resolution and improvement (improvement ≥1 grade from worst grade as of the latest assessment) in pts with ongoing symptoms at end of treatment (EoT), rate of secondary malignancies, and incidence and outcomes of pregnancies among pts and their partners.
Results
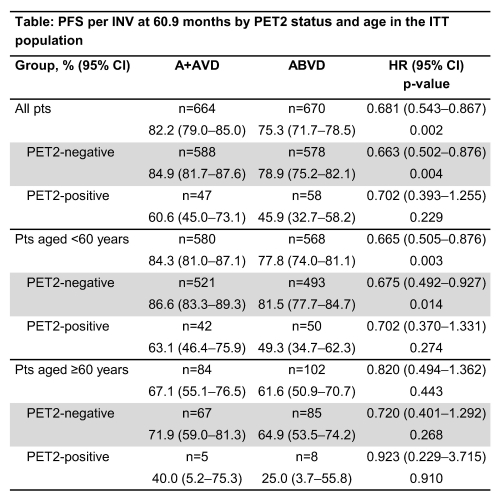
At a median follow-up of 60.9 months (95% confidence interval [CI] 55.2–56.7), estimated 5-year PFS per INV rates were 82.2% (95% CI 79.0–85.0) for A+AVD and 75.3% (95% CI 71.7–78.5) for ABVD. A+AVD demonstrated favorable PFS per INV vs ABVD (hazard ratio [HR] 0.681; 95% CI 0.534–0.867; p=0.002) (Table). Estimated 5-year PFS with A+AVD vs ABVD in the ITT population was 84.9% vs 78.9% in PET2-negative pts (HR 0.663; 95% CI 0.502–0.876; p=0.004) and 60.6% vs 45.9% in PET2-positive pts (HR 0.702; 95% CI 0.393–1.255; p=0.229). In the A+AVD and ABVD arms, 85% and 86% of pts with treatment-emergent PN had complete resolution or improvement of symptoms, respectively. Median time to complete resolution of ongoing PN events at EoT was 34 weeks (range 0–262) in the A+AVD arm and 16 weeks (range 0–267) in the ABVD arm; median time to improvement was 49 weeks (range 8–270) and 12 weeks (range 2–70), respectively. Maximum severity of ongoing PN was grade 1 (17%), grade 2 (9%), grade 3 (3%), and grade 4 (<1%) in the A+AVD arm (total=29%), and grade 1 (14%), 2 (6%) or 3 (1%) in the ABVD arm (total=21%). Secondary malignancies occurred in 19 and 29 pts in A+AVD and ABVD arms, respectively. In total, 131 pregnancies were reported; both arms showed similar proportions of ongoing pregnancies or live births in female pts (87% and 75% in the A+AVD and ABVD arms, respectively).
Conclusion
After 60.9 months’ median follow-up, robust and durable treatment benefits improvements that were independent of disease stage, risk factor score, and PET2 status were seen with A+AVD vs ABVD. Treatment adaptation by interim PET2 status was not required for A+AVD and bleomycin exposure was avoided. Treatment with A+AVD provides sustained PFS benefits and a manageable safety profile with symptoms of PN improving or resolving over time and similar pregnancy rates in both treatment arms, suggesting that A+AVD is an attractive treatment option for all pts with previously untreated Stage III or IV cHL.
Keyword(s): CD30, Hodgkin's lymphoma, Phase III, Targeted therapy
Abstract: S205
Type: Oral Presentation
Session title: Hodgkin lymphoma - Clinical
Background
Historically, nearly all relapses in classical Hodgkin lymphoma (cHL) occur within 5 years of treatment (Radford, BMJ 1997). In the phase 3 ECHELON-1 study (NCT01712490) in patients (pts) with newly-diagnosed Stage III/IV cHL, treatment with brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine (A+AVD) significantly improved modified progression-free survival (PFS) per independent review facility vs doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) (Connors et al, NEJM 2018). After 3 and 4 years’ follow-up, sustained PFS per investigator (INV) benefits were seen with A+AVD vs ABVD in the intent-to-treat (ITT) population as well as across most key pt subgroups (Bartlett, Blood 2019; Straus, Blood 2020). These improvements were irrespective of interim positron emission tomography (PET) scan status, disease stage, and baseline disease risk factor score.
Aims
We report updated efficacy and safety results after 5-years’ follow-up of the ECHELON-1 study.
Methods
Pts with previously untreated Stage III/IV cHL were randomized to receive ≤6 cycles of intravenous A+AVD (n=664) or ABVD (n=670) on days 1 and 15 of a 28-day cycle. After cycle 2, pts had an interim PET scan (PET2). Analyses were performed after extended follow-up (cutoff date Sept’ 18, 2020) to assess PFS per INV, peripheral neuropathy (PN) resolution and improvement (improvement ≥1 grade from worst grade as of the latest assessment) in pts with ongoing symptoms at end of treatment (EoT), rate of secondary malignancies, and incidence and outcomes of pregnancies among pts and their partners.
Results
At a median follow-up of 60.9 months (95% confidence interval [CI] 55.2–56.7), estimated 5-year PFS per INV rates were 82.2% (95% CI 79.0–85.0) for A+AVD and 75.3% (95% CI 71.7–78.5) for ABVD. A+AVD demonstrated favorable PFS per INV vs ABVD (hazard ratio [HR] 0.681; 95% CI 0.534–0.867; p=0.002) (Table). Estimated 5-year PFS with A+AVD vs ABVD in the ITT population was 84.9% vs 78.9% in PET2-negative pts (HR 0.663; 95% CI 0.502–0.876; p=0.004) and 60.6% vs 45.9% in PET2-positive pts (HR 0.702; 95% CI 0.393–1.255; p=0.229). In the A+AVD and ABVD arms, 85% and 86% of pts with treatment-emergent PN had complete resolution or improvement of symptoms, respectively. Median time to complete resolution of ongoing PN events at EoT was 34 weeks (range 0–262) in the A+AVD arm and 16 weeks (range 0–267) in the ABVD arm; median time to improvement was 49 weeks (range 8–270) and 12 weeks (range 2–70), respectively. Maximum severity of ongoing PN was grade 1 (17%), grade 2 (9%), grade 3 (3%), and grade 4 (<1%) in the A+AVD arm (total=29%), and grade 1 (14%), 2 (6%) or 3 (1%) in the ABVD arm (total=21%). Secondary malignancies occurred in 19 and 29 pts in A+AVD and ABVD arms, respectively. In total, 131 pregnancies were reported; both arms showed similar proportions of ongoing pregnancies or live births in female pts (87% and 75% in the A+AVD and ABVD arms, respectively).

Conclusion
After 60.9 months’ median follow-up, robust and durable treatment benefits improvements that were independent of disease stage, risk factor score, and PET2 status were seen with A+AVD vs ABVD. Treatment adaptation by interim PET2 status was not required for A+AVD and bleomycin exposure was avoided. Treatment with A+AVD provides sustained PFS benefits and a manageable safety profile with symptoms of PN improving or resolving over time and similar pregnancy rates in both treatment arms, suggesting that A+AVD is an attractive treatment option for all pts with previously untreated Stage III or IV cHL.
Keyword(s): CD30, Hodgkin's lymphoma, Phase III, Targeted therapy


