
Contributions
Abstract: S203
Type: Oral Presentation
Session title: Novel therapies and targets in MPN
Background
Aims
Determine progression-free survival (PFS) and overall survival (OS) with FEDR 400 mg/d in JAKARTA and JAKARTA2.
Methods
Both trials enrolled pts aged ≥18 years with INT- or high-risk MF, platelet counts ≥50 ×109/L, and ECOG PS ≤2. In JAKARTA, pts were randomized 1:1:1 to receive 1L FEDR 400 mg/d, FEDR 500 mg/d, or PBO for ≥6 4-week cycles (randomized Tx period). At the end of 6 Tx cycles (or earlier in cases of disease progression [PD]), pts randomized to PBO could cross-over to FEDR. In JAKARTA2, all pts with MF resistant or intolerant to prior RUX (per investigator assessment) received FEDR 400 mg/d (starting dose). Pts in both trials could continue to receive FEDR until PD or unacceptable toxicity. These analyses include pts who received FEDR 400 mg/d in each study and pts in JAKARTA randomized to PBO.
PFS was the time from randomization to PD (IWG-MRT criteria) or death (in the absence of PD/death, PFS was censored at the date of the last valid assessment), and OS was the time from randomization to death. PFS/OS were estimated using Kaplan-Meier methods. Importantly, PFS/OS results for pts in the JAKARTA PBO arm include time after crossover to FEDR. Due to a clinical hold in Nov 2013 (later lifted), both trials were terminated early and survival follow-up stopped; ongoing pts were censored at the time of the clinical hold.
Results
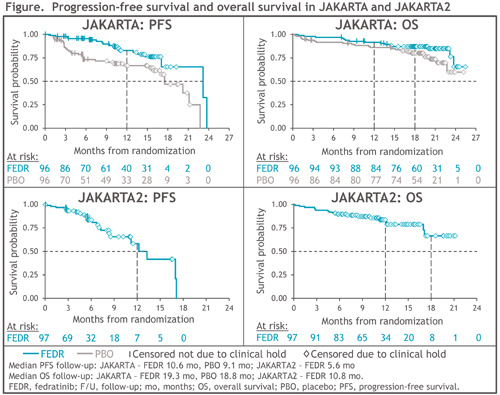
In JAKARTA, 96 pts were randomized to FEDR 400 mg/d and 96 to PBO; 71 pts (74%) in the PBO arm crossed-over to FEDR on-study. At study termination, survival follow-up was halted for 74 (77%) and 65 (68%) pts randomized to FEDR 400 mg/d and PBO, respectively. PFS was significantly prolonged with FEDR vs PBO: median 23.2 vs 17.5 mo, respectively (HR 0.42 [95%CI 0.23, 0.76]; P=0.004), and 1-year PFS rates were 83% vs 67% (Figure). Median OS was not reached (NR) in either Tx arm (HR 0.57 [0.30, 1.10]; P=0.094); 1-year and 18-mo survival rates in the FEDR arm were 92% and 87%, respectively, and in the PBO arm were 86% and 80%.
JAKARTA2 enrolled 97 pts, and 79 (81%) were censored for survival at study termination. Median PFS was 13.3 mo (95%CI 8.4, 17.1) and 1-year PFS was 59%. Median OS was NR (95%CI 17.1, NR) (Figure), and 1-year and 18-mo survival rates were 84% and 67%, respectively.
Conclusion
FEDR significantly improved PFS vs PBO as 1L MF Tx. Separation of PFS/OS curves between FEDR and PBO in JAKARTA, despite FEDR Tx in the PBO arm after Tx crossover, suggests pts may derive greater benefit from early FEDR use. For pts in JAKARTA2 treated with FEDR after prior RUX, median PFS, median OS, and 1-year survival rate compare favorably with outcomes after RUX discontinuation from a recent analysis of similar pts with MF included in large healthcare databases (6.0 mo, 11.1 mo, and 47%, respectively) (Mascarenhas, 2020). These survival outcomes were greatly impacted by the FEDR clinical hold. The ongoing FREEDOM and FREEDOM2 trials will further assess survival with FEDR in pts with MF.
Keyword(s): Fedratinib, Myelofibrosis, Progression, Survival
Abstract: S203
Type: Oral Presentation
Session title: Novel therapies and targets in MPN
Background
Aims
Determine progression-free survival (PFS) and overall survival (OS) with FEDR 400 mg/d in JAKARTA and JAKARTA2.
Methods
Both trials enrolled pts aged ≥18 years with INT- or high-risk MF, platelet counts ≥50 ×109/L, and ECOG PS ≤2. In JAKARTA, pts were randomized 1:1:1 to receive 1L FEDR 400 mg/d, FEDR 500 mg/d, or PBO for ≥6 4-week cycles (randomized Tx period). At the end of 6 Tx cycles (or earlier in cases of disease progression [PD]), pts randomized to PBO could cross-over to FEDR. In JAKARTA2, all pts with MF resistant or intolerant to prior RUX (per investigator assessment) received FEDR 400 mg/d (starting dose). Pts in both trials could continue to receive FEDR until PD or unacceptable toxicity. These analyses include pts who received FEDR 400 mg/d in each study and pts in JAKARTA randomized to PBO.
PFS was the time from randomization to PD (IWG-MRT criteria) or death (in the absence of PD/death, PFS was censored at the date of the last valid assessment), and OS was the time from randomization to death. PFS/OS were estimated using Kaplan-Meier methods. Importantly, PFS/OS results for pts in the JAKARTA PBO arm include time after crossover to FEDR. Due to a clinical hold in Nov 2013 (later lifted), both trials were terminated early and survival follow-up stopped; ongoing pts were censored at the time of the clinical hold.
Results
In JAKARTA, 96 pts were randomized to FEDR 400 mg/d and 96 to PBO; 71 pts (74%) in the PBO arm crossed-over to FEDR on-study. At study termination, survival follow-up was halted for 74 (77%) and 65 (68%) pts randomized to FEDR 400 mg/d and PBO, respectively. PFS was significantly prolonged with FEDR vs PBO: median 23.2 vs 17.5 mo, respectively (HR 0.42 [95%CI 0.23, 0.76]; P=0.004), and 1-year PFS rates were 83% vs 67% (Figure). Median OS was not reached (NR) in either Tx arm (HR 0.57 [0.30, 1.10]; P=0.094); 1-year and 18-mo survival rates in the FEDR arm were 92% and 87%, respectively, and in the PBO arm were 86% and 80%.
JAKARTA2 enrolled 97 pts, and 79 (81%) were censored for survival at study termination. Median PFS was 13.3 mo (95%CI 8.4, 17.1) and 1-year PFS was 59%. Median OS was NR (95%CI 17.1, NR) (Figure), and 1-year and 18-mo survival rates were 84% and 67%, respectively.

Conclusion
FEDR significantly improved PFS vs PBO as 1L MF Tx. Separation of PFS/OS curves between FEDR and PBO in JAKARTA, despite FEDR Tx in the PBO arm after Tx crossover, suggests pts may derive greater benefit from early FEDR use. For pts in JAKARTA2 treated with FEDR after prior RUX, median PFS, median OS, and 1-year survival rate compare favorably with outcomes after RUX discontinuation from a recent analysis of similar pts with MF included in large healthcare databases (6.0 mo, 11.1 mo, and 47%, respectively) (Mascarenhas, 2020). These survival outcomes were greatly impacted by the FEDR clinical hold. The ongoing FREEDOM and FREEDOM2 trials will further assess survival with FEDR in pts with MF.
Keyword(s): Fedratinib, Myelofibrosis, Progression, Survival


