
Contributions
Abstract: S186
Type: Oral Presentation
Session title: New diagnostic and therapeutic approaches in multiple myeloma and AL amyloidosis
Background
Isa is an approved monoclonal antibody that binds to a specific epitope on the CD38 receptor. The Phase 3 ICARIA-MM study (NCT02990338) demonstrated significantly improved progression-free survival (PFS) with Isa-Pd vs Pd (P=0.001) and a manageable safety profile (Attal M, et al. Lancet 2019;394:2096-2107).
Aims
Here we report updated ICARIA results.
Methods
Pts with RRMM (N=307) who have received ≥2 prior therapies, including lenalidomide (Len) and a proteasome inhibitor (PI), were randomized to Isa-Pd (n=154) or Pd (n=153). Isa was administered intravenously at 10 mg/kg weekly for 4 weeks, and every other week thereafter. This preplanned second interim analysis assessed longer term outcomes, including time to next treatment (TTNT), overall survival (OS), time from randomization to disease progression on first subsequent therapy or death (PFS2), and safety.
Results
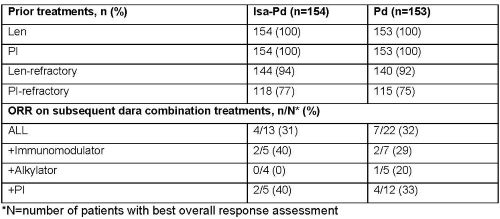
Pts had a median of 3 prior lines of therapy (IQR 2–4; table). As of Oct 1, 2020 (median follow-up, 35.3 months [mo]), 27 Isa-Pd pts (18%) vs 12 Pd pts (8%) were still on treatment; 60% vs 72% had proceeded to subsequent therapy. Median TTNT was 15.5 mo with Isa-Pd vs 8.9 mo with Pd (hazard ratio [HR] 0.56; 95% confidence interval [CI] 0.42–0.74; P<0.0001); 24% vs 58% of pts with subsequent therapy received daratumumab (dara). Overall response rate (ORR) in subsequent treatment with dara monotherapy was higher after Pd (38%) than Isa-Pd (14%), but was similar (32% vs 31%) with dara combination therapy (table). Median PFS2 in the intent-to-treat population was 17.5 mo with Isa-Pd vs 12.9 mo with Pd (HR 0.76; 95% CI 0.58–0.99; P=0.0202). Median OS was 24.6 mo with Isa-Pd vs 17.7 mo with Pd (HR 0.76; 95% CI 0.58–1.01; one-sided P=0.0280). Median treatment duration was 47.6 weeks (range 1.3–171.6) with Isa-Pd vs 24.0 weeks (range 1.0–168.6) with Pd. Serious treatment-emergent adverse event (TEAEs) were seen in 73% of Isa-Pd pts vs 60% of Pd pts. Most frequent non-hematologic TEAEs (any grade) with Isa-Pd were infusion reaction (38%), upper respiratory tract infection (34%), and diarrhea (30%). Grade 3–4 neutropenia (85% vs 71%) and thrombocytopenia (34% vs 25%) were more frequent with Isa-Pd than with Pd.
Conclusion
Isa-Pd demonstrates a significant improvement in TTNT and PFS2 compared with Pd. A strong trend in OS benefit was also seen in the Isa-Pd arm, with approximately 7 mo improvement in median OS. The overall safety profile remains unchanged from prior analyses.
Keyword(s): CD38, Monoclonal antibody, Multiple myeloma, Phase III
Abstract: S186
Type: Oral Presentation
Session title: New diagnostic and therapeutic approaches in multiple myeloma and AL amyloidosis
Background
Isa is an approved monoclonal antibody that binds to a specific epitope on the CD38 receptor. The Phase 3 ICARIA-MM study (NCT02990338) demonstrated significantly improved progression-free survival (PFS) with Isa-Pd vs Pd (P=0.001) and a manageable safety profile (Attal M, et al. Lancet 2019;394:2096-2107).
Aims
Here we report updated ICARIA results.
Methods
Pts with RRMM (N=307) who have received ≥2 prior therapies, including lenalidomide (Len) and a proteasome inhibitor (PI), were randomized to Isa-Pd (n=154) or Pd (n=153). Isa was administered intravenously at 10 mg/kg weekly for 4 weeks, and every other week thereafter. This preplanned second interim analysis assessed longer term outcomes, including time to next treatment (TTNT), overall survival (OS), time from randomization to disease progression on first subsequent therapy or death (PFS2), and safety.
Results
Pts had a median of 3 prior lines of therapy (IQR 2–4; table). As of Oct 1, 2020 (median follow-up, 35.3 months [mo]), 27 Isa-Pd pts (18%) vs 12 Pd pts (8%) were still on treatment; 60% vs 72% had proceeded to subsequent therapy. Median TTNT was 15.5 mo with Isa-Pd vs 8.9 mo with Pd (hazard ratio [HR] 0.56; 95% confidence interval [CI] 0.42–0.74; P<0.0001); 24% vs 58% of pts with subsequent therapy received daratumumab (dara). Overall response rate (ORR) in subsequent treatment with dara monotherapy was higher after Pd (38%) than Isa-Pd (14%), but was similar (32% vs 31%) with dara combination therapy (table). Median PFS2 in the intent-to-treat population was 17.5 mo with Isa-Pd vs 12.9 mo with Pd (HR 0.76; 95% CI 0.58–0.99; P=0.0202). Median OS was 24.6 mo with Isa-Pd vs 17.7 mo with Pd (HR 0.76; 95% CI 0.58–1.01; one-sided P=0.0280). Median treatment duration was 47.6 weeks (range 1.3–171.6) with Isa-Pd vs 24.0 weeks (range 1.0–168.6) with Pd. Serious treatment-emergent adverse event (TEAEs) were seen in 73% of Isa-Pd pts vs 60% of Pd pts. Most frequent non-hematologic TEAEs (any grade) with Isa-Pd were infusion reaction (38%), upper respiratory tract infection (34%), and diarrhea (30%). Grade 3–4 neutropenia (85% vs 71%) and thrombocytopenia (34% vs 25%) were more frequent with Isa-Pd than with Pd.

Conclusion
Isa-Pd demonstrates a significant improvement in TTNT and PFS2 compared with Pd. A strong trend in OS benefit was also seen in the Isa-Pd arm, with approximately 7 mo improvement in median OS. The overall safety profile remains unchanged from prior analyses.
Keyword(s): CD38, Monoclonal antibody, Multiple myeloma, Phase III


