
Contributions
Abstract: S185
Type: Oral Presentation
Session title: New diagnostic and therapeutic approaches in multiple myeloma and AL amyloidosis
Background
There is great expectation in liquid biopsies to stratify patients with cancer. These could be particularly valuable in MM where despite patchy infiltration and likelihood of extramedullary disease, quantification of tumor burden continues being performed in bone marrow (BM). However, there are no large studies analyzing if liquid biopsies using next-generation methods yield superior prognostic information than conventional cytology in BM.
Aims
To establish the clinical significance of CTC burden in peripheral blood (PB) and to define cutoffs for risk stratification of newly-diagnosed MM patients.
Methods
This study included 375 transplant-eligible patients enrolled in the GEM2012MENOS65 clinical trial and treated with six induction cycles of VRD followed by autologous transplant and consolidation with two VRD courses. Afterwards, patients received Rd (with or without ixazomib) for two years and either stopped maintenance if MRD negative or continued three more years if MRD positive (GEM2014MAIN). EuroFlow next-generation flow was used to evaluate CTCs in PB at diagnosis and MRD in BM throughout treatment. Cutoffs were defined using maximally selected rank statistics considering PFS. Median follow-up is of 5 years.
Results
CTC were detected in 345/375 (92%) of patients. There was a modest correlation between the percentage of CTCs in PB and tumor burden in BM by morphology (R2=0.35, p<0.0001) and flow cytometry (R2=0.41, p<0.0001). These data suggests that CTC egression is not directly related with tumor burden in BM.
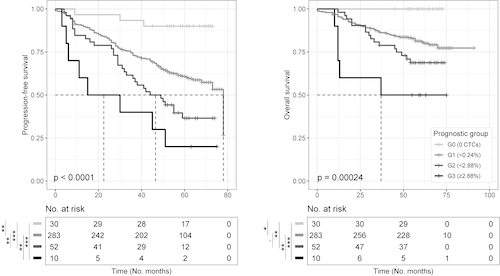
Patients stratification into 4 subgroups according to the percentage of CTC (Group 0: 0%, Group 1: >0% to <0.24%, Group 2: ≥0.24% to <2.88%, and Group 3: ≥2.88%) resulted in significant differences in PFS (median not reached [NR] vs 78, 47 and 23 months, and hazard ratios of 4.9, 8.8 and 15.8, respectively; p<0.0001) and overall survival ([OS] 100%, 81%, 69% and 50% at 5 years, p=0.0002). In a multivariable analysis including quantification of tumor burden in PB using NGF and in BM by morphology and flow cytometry, the percentage of CTC was selected as the only independent prognostic factor for PFS (p=0.001). In a subsequent multivariable analysis including the percentage of CTC in PB, ISS, LDH and FISH cytogenetics, quantification of CTC was selected as the most relevant prognostic factor for PFS. Indeed, risk stratification according to the CTC cutoffs previously defined was prognostic in all risk groups including patients with standard-risk FISH (median PFS NR in Groups 0 & 1 vs 44 and 30 months in Groups 2 & 3, respectively: p=0.005).
We further investigated if deep responses to treatment were able to abrogate the poor prognosis of elevated CTC at diagnosis. Patients in Groups 2 & 3 showed significantly inferior PFS (median of 59 and 48 months) than cases in Groups 0 & 1 (median NR; p=0.004) despite achieving CR. By contrast, there were no significant differences in PFS of MRD negative patients stratified according to the percentage of CTC (p=0.017). Patients with 0% CTC and negative MRD before maintenance showed outstanding PFS and OS rates of 94% and 100% at 5 years.
Conclusion
Evaluation of CTC in PB outperformed quantification of tumor burden in BM and was the most relevant prognostic factor at baseline. Attaining undetectable MRD as opposed to CR should be considered as the treatment endpoint in patients with elevated percentages of CTC in PB. Patients with undetectable CTC at diagnosis and undetectable MRD after treatment intensification showed long-term survival with fixed duration maintenance therapy.
Keyword(s): Bone marrow biopsy, Minimal residual disease (MRD), Peripheral blood, Survival prediction
Abstract: S185
Type: Oral Presentation
Session title: New diagnostic and therapeutic approaches in multiple myeloma and AL amyloidosis
Background
There is great expectation in liquid biopsies to stratify patients with cancer. These could be particularly valuable in MM where despite patchy infiltration and likelihood of extramedullary disease, quantification of tumor burden continues being performed in bone marrow (BM). However, there are no large studies analyzing if liquid biopsies using next-generation methods yield superior prognostic information than conventional cytology in BM.
Aims
To establish the clinical significance of CTC burden in peripheral blood (PB) and to define cutoffs for risk stratification of newly-diagnosed MM patients.
Methods
This study included 375 transplant-eligible patients enrolled in the GEM2012MENOS65 clinical trial and treated with six induction cycles of VRD followed by autologous transplant and consolidation with two VRD courses. Afterwards, patients received Rd (with or without ixazomib) for two years and either stopped maintenance if MRD negative or continued three more years if MRD positive (GEM2014MAIN). EuroFlow next-generation flow was used to evaluate CTCs in PB at diagnosis and MRD in BM throughout treatment. Cutoffs were defined using maximally selected rank statistics considering PFS. Median follow-up is of 5 years.
Results
CTC were detected in 345/375 (92%) of patients. There was a modest correlation between the percentage of CTCs in PB and tumor burden in BM by morphology (R2=0.35, p<0.0001) and flow cytometry (R2=0.41, p<0.0001). These data suggests that CTC egression is not directly related with tumor burden in BM.
Patients stratification into 4 subgroups according to the percentage of CTC (Group 0: 0%, Group 1: >0% to <0.24%, Group 2: ≥0.24% to <2.88%, and Group 3: ≥2.88%) resulted in significant differences in PFS (median not reached [NR] vs 78, 47 and 23 months, and hazard ratios of 4.9, 8.8 and 15.8, respectively; p<0.0001) and overall survival ([OS] 100%, 81%, 69% and 50% at 5 years, p=0.0002). In a multivariable analysis including quantification of tumor burden in PB using NGF and in BM by morphology and flow cytometry, the percentage of CTC was selected as the only independent prognostic factor for PFS (p=0.001). In a subsequent multivariable analysis including the percentage of CTC in PB, ISS, LDH and FISH cytogenetics, quantification of CTC was selected as the most relevant prognostic factor for PFS. Indeed, risk stratification according to the CTC cutoffs previously defined was prognostic in all risk groups including patients with standard-risk FISH (median PFS NR in Groups 0 & 1 vs 44 and 30 months in Groups 2 & 3, respectively: p=0.005).
We further investigated if deep responses to treatment were able to abrogate the poor prognosis of elevated CTC at diagnosis. Patients in Groups 2 & 3 showed significantly inferior PFS (median of 59 and 48 months) than cases in Groups 0 & 1 (median NR; p=0.004) despite achieving CR. By contrast, there were no significant differences in PFS of MRD negative patients stratified according to the percentage of CTC (p=0.017). Patients with 0% CTC and negative MRD before maintenance showed outstanding PFS and OS rates of 94% and 100% at 5 years.

Conclusion
Evaluation of CTC in PB outperformed quantification of tumor burden in BM and was the most relevant prognostic factor at baseline. Attaining undetectable MRD as opposed to CR should be considered as the treatment endpoint in patients with elevated percentages of CTC in PB. Patients with undetectable CTC at diagnosis and undetectable MRD after treatment intensification showed long-term survival with fixed duration maintenance therapy.
Keyword(s): Bone marrow biopsy, Minimal residual disease (MRD), Peripheral blood, Survival prediction


