
Contributions
Abstract: S184
Type: Oral Presentation
Session title: New treatment strategies for newly diagnosed multiple myeloma
Background
MRD status is the most powerful prognostic factor in NDMM and sustained MRD negativity is a surrogate for prolonged survival. Interest is growing for using MRD to guide treatment intensity and duration, particularly during maintenance. Paradoxically, MRD data in this setting is scarce and knowledge of the volatility of MRD status throughout treatment is limited.
Aims
To present a pooled analysis of the Phase 3 TOURMALINE-MM3 and -MM4 trials, the largest maintenance dataset ever reported, to evaluate the impact of evolving MRD kinetics on progression-free survival (PFS) in NDMM pts receiving ixa or placebo (pbo) maintenance.
Methods
Pts with ≥partial response (PR) following proteasome inhibitor and/or immunomodulatory drug induction plus a single transplant (MM3) or 6–12 months (mos) of standard-of-care induction therapy (MM4) were randomized (3:2) to oral ixa 3 mg (increased to 4 mg if tolerated after 4 cycles) or pbo in 28-day cycles for up to 2 years (or 26 cycles). PFS from randomization was the primary endpoint; correlation of MRD status with outcomes was a prespecified secondary endpoint in both trials. 2077 bone marrow aspirates were collected from all pts in complete response (CR; MM3 & MM4) or very good PR (MM3 only) at screening, cycle 13, and cycle 26/end of treatment, and at time of new suspected CR (MM3 & MM4). MRD status was determined using a validated 8-color flow cytometry approach standardized across three geographic regions (38 countries); median limit of detection was 7.4x10-6. Pts with
Results
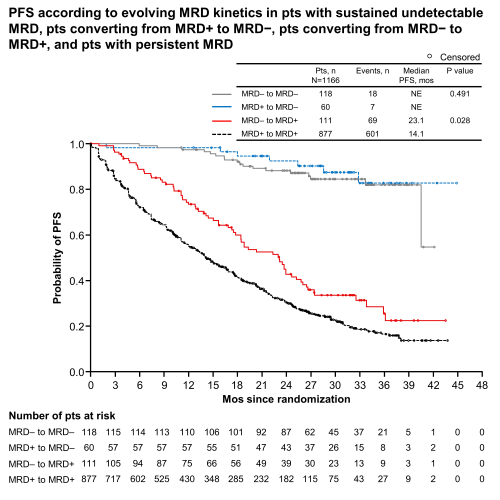
At screening, MRD status was available for 1280 pts: 262 were MRD− (ixa n=161/767 [21%]; pbo n=101/513 [20%]) and 1018 MRD+ (ixa n=606 [79%]; pbo n=412 [80%]). In pts who were MRD+ at study entry, there was significant PFS benefit for ixa vs pbo (median 18.8 vs 11.6 mos, hazard ratio [HR] 0.65, 95% CI 0.55–0.76, p<.001). 10% of pts showed sustained MRD negativity, 5% converted from MRD+ to MRD−, 10% from MRD− to MRD+ and 75% had persistent MRD. Median PFS (mPFS) was not reached in pts with sustained MRD negativity (Figure; ixa vs pbo HR 0.89, p=.815). mPFS was also not reached in pts converting from MRD+ to MRD− during treatment; there was significant benefit with ixa vs pbo (HR 0.14, p=.007). By comparison, mPFS was inferior in pts converting from MRD− to MRD+ (23.1 mos) and dismal in pts with persistent MRD (14.1 mos); mPFS with ixa vs pbo was 23.2 vs 19.3 mos (HR 0.81, p=.411) and 17.5 vs 11.1 mos (HR 0.68, p<.001) in these groups, respectively. Other disease characteristics had no prognostic value once pts were stratified into these four MRD kinetics subgroups. Overall, failure to achieve/sustain MRD− status post-screening resulted in a >8-fold increased risk of progression and/or death (HR 8.20, 95% CI 5.49–12.2, p<.001).
Conclusion
This large dataset demonstrated that the prognostic value of MRD status at the start of maintenance can be enhanced by measuring MRD kinetics during treatment. Our results support the achievement and sustainability of MRD negativity as a treatment endpoint in the maintenance setting and showed poor outcomes in MRD− pts converting to MRD+, underscoring the value of serial MRD assessments to anticipate relapse and guide treatment decisions. Accordingly, ixa showed significant PFS benefit vs pbo in pts who were MRD+ at study entry and pts with persistent MRD.
Keyword(s): Maintenance, Minimal residual disease (MRD), Multiple myeloma, Proteasome inhibitor
Abstract: S184
Type: Oral Presentation
Session title: New treatment strategies for newly diagnosed multiple myeloma
Background
MRD status is the most powerful prognostic factor in NDMM and sustained MRD negativity is a surrogate for prolonged survival. Interest is growing for using MRD to guide treatment intensity and duration, particularly during maintenance. Paradoxically, MRD data in this setting is scarce and knowledge of the volatility of MRD status throughout treatment is limited.
Aims
To present a pooled analysis of the Phase 3 TOURMALINE-MM3 and -MM4 trials, the largest maintenance dataset ever reported, to evaluate the impact of evolving MRD kinetics on progression-free survival (PFS) in NDMM pts receiving ixa or placebo (pbo) maintenance.
Methods
Pts with ≥partial response (PR) following proteasome inhibitor and/or immunomodulatory drug induction plus a single transplant (MM3) or 6–12 months (mos) of standard-of-care induction therapy (MM4) were randomized (3:2) to oral ixa 3 mg (increased to 4 mg if tolerated after 4 cycles) or pbo in 28-day cycles for up to 2 years (or 26 cycles). PFS from randomization was the primary endpoint; correlation of MRD status with outcomes was a prespecified secondary endpoint in both trials. 2077 bone marrow aspirates were collected from all pts in complete response (CR; MM3 & MM4) or very good PR (MM3 only) at screening, cycle 13, and cycle 26/end of treatment, and at time of new suspected CR (MM3 & MM4). MRD status was determined using a validated 8-color flow cytometry approach standardized across three geographic regions (38 countries); median limit of detection was 7.4x10-6. Pts with
Results
At screening, MRD status was available for 1280 pts: 262 were MRD− (ixa n=161/767 [21%]; pbo n=101/513 [20%]) and 1018 MRD+ (ixa n=606 [79%]; pbo n=412 [80%]). In pts who were MRD+ at study entry, there was significant PFS benefit for ixa vs pbo (median 18.8 vs 11.6 mos, hazard ratio [HR] 0.65, 95% CI 0.55–0.76, p<.001). 10% of pts showed sustained MRD negativity, 5% converted from MRD+ to MRD−, 10% from MRD− to MRD+ and 75% had persistent MRD. Median PFS (mPFS) was not reached in pts with sustained MRD negativity (Figure; ixa vs pbo HR 0.89, p=.815). mPFS was also not reached in pts converting from MRD+ to MRD− during treatment; there was significant benefit with ixa vs pbo (HR 0.14, p=.007). By comparison, mPFS was inferior in pts converting from MRD− to MRD+ (23.1 mos) and dismal in pts with persistent MRD (14.1 mos); mPFS with ixa vs pbo was 23.2 vs 19.3 mos (HR 0.81, p=.411) and 17.5 vs 11.1 mos (HR 0.68, p<.001) in these groups, respectively. Other disease characteristics had no prognostic value once pts were stratified into these four MRD kinetics subgroups. Overall, failure to achieve/sustain MRD− status post-screening resulted in a >8-fold increased risk of progression and/or death (HR 8.20, 95% CI 5.49–12.2, p<.001).

Conclusion
This large dataset demonstrated that the prognostic value of MRD status at the start of maintenance can be enhanced by measuring MRD kinetics during treatment. Our results support the achievement and sustainability of MRD negativity as a treatment endpoint in the maintenance setting and showed poor outcomes in MRD− pts converting to MRD+, underscoring the value of serial MRD assessments to anticipate relapse and guide treatment decisions. Accordingly, ixa showed significant PFS benefit vs pbo in pts who were MRD+ at study entry and pts with persistent MRD.
Keyword(s): Maintenance, Minimal residual disease (MRD), Multiple myeloma, Proteasome inhibitor


