
Contributions
Abstract: S169
Type: Oral Presentation
Session title: Novel targets in MDS
Background
The GATA2 gene, a DNA binding transcription factor with two zinc finger domains, is crucial for the regulation of hematopoietic stem cell proliferation, differentiation and maintenance (Wlodarski et al. Semin Hematol 2017). GATA2 mutations are seen in myeloid neoplasms (MN) with different domains being associated with different leukemogenic
Aims
To describe patients (pts) with GATA2-mutated MN.
Methods
In this study, we included pts with GATA2 mutations diagnosed with MN between 2015 and 2020 at Mayo Clinic. Pts characteristics were obtained from medical records. Our institution’s next-generation sequencing (NGS) panel (OncoHeme) was analyzed to detect mutations in 42 genes recurrently mutated in MN. JMP® Pro 14.1.0 Software was used for statistical analysis
Results
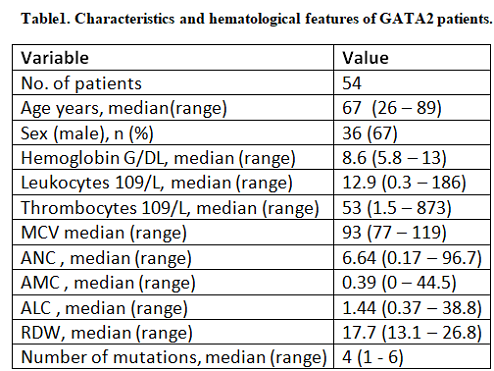
Out of 3,872 pts who underwent NGS testing, a total of 54 pts were identified harboring 64 GATA2 mutations in four diagnostic groups; 15 (27.7%) myelodysplastic syndromes (MDS), 16(29.6%) myelodysplastic/myeloproliferative neoplasms overlap (MDS/MPN), 9 (16.6%) myeloproliferative neoplasms (MPN) and 14 (25.9%) acute myeloid leukemia (AML). The median age was 67 years, 67% were males (Table 1).
A) GATA2 mutations characteristics and co-mutations: Median GATA2 mutation VAF was 31% in the total cohort (range 5-74%), with 28%, 37%, 34%, and 39% (p= .6) seen in MDS, MDS/MPN, MPN, and AML, respectively. 31 (57%) mutations clustered in the zinc finger 2 domain (ZF2), 9 (17%) in the ZF1 domain and 14 (26%) were outside the ZF domains (non-ZF). ZF1-mutated pts had lower mean VAF compared to ZF2 pts (22% vs. 38%, p=.03), and the majority occurred in AML cases (6/9, 67%), with the most common mutation type being missense (78%). There was a significant association between mutation type and location (p <.0001). The median number of mutations per pts was 4. We identified 28 co-mutations in 52 (96%) pts. ASXL1 (60%), SRSF2 (33%) and RUNX1 (19%) were the most common co-mutations. ASXL1 was frequently co-mutated with GATA2-ZF2 (74%) and non-ZF (22.6%) (only 1 co-mutated with GATA2-ZF1). ASXL1 was also more commonly co-mutated in chronic MN compared to AML (94% vs. 6%, p=.0001). KIT, CEBPA and PTPN11 were co-mutated only in ZF1-mutated pts. KIT, FLT3, PTPN11, CEBPA were co-mutated only in AML, whereas CALR was co-mutated only in MPN.
B) Survival outcome: After a median follow-up time of 26.4 months, 34 (63%) pts died with a median OS of 23.9, 22.4, 20.6, and 5.6 months in MDS, MDS/MPN, MPN, and AML pts; respectively. After excluding AML pts for survival analysis, we found that pts with several GATA2 mutations had poorer median OS (4.9 vs. 24.5 months, p=0.03) compared to those with single GATA2 mutation. MDS pts with chromatin modification co-mutations (BCOR, EZH2, ASXL1, KDM6A) (17 vs. 30 months, p=.04) or frameshift (FS) mutation (10.3 vs. 27.3 months, p=.05) had worse median OS compared to those without chromatin modification co-mutation or non-FS mutation, respectively. MDS/MPN overlap pts with RUNX1 co-mutation had worse median OS (2.2 vs. 25.7 months, p=.0004) than their un-mutated counterparts. There was no difference in median OS between GATA2-ZF1, ZF2, and non-ZF (30 vs. 20.6 vs. 22.4 months, p= .7).
Conclusion
We describe the spectrum and characteristics of GATA2 mutations across myeloid neoplasms. There was an impact of different GATA2-mutation types, single vs several GATA2 mutations, and co-mutations pattern on survival. Our work was limited by the small number of patients and inability to completely rule out germline mutation possibility.
Keyword(s): GATA-2, Myeloid malignancies, Outcome
Abstract: S169
Type: Oral Presentation
Session title: Novel targets in MDS
Background
The GATA2 gene, a DNA binding transcription factor with two zinc finger domains, is crucial for the regulation of hematopoietic stem cell proliferation, differentiation and maintenance (Wlodarski et al. Semin Hematol 2017). GATA2 mutations are seen in myeloid neoplasms (MN) with different domains being associated with different leukemogenic
Aims
To describe patients (pts) with GATA2-mutated MN.
Methods
In this study, we included pts with GATA2 mutations diagnosed with MN between 2015 and 2020 at Mayo Clinic. Pts characteristics were obtained from medical records. Our institution’s next-generation sequencing (NGS) panel (OncoHeme) was analyzed to detect mutations in 42 genes recurrently mutated in MN. JMP® Pro 14.1.0 Software was used for statistical analysis
Results
Out of 3,872 pts who underwent NGS testing, a total of 54 pts were identified harboring 64 GATA2 mutations in four diagnostic groups; 15 (27.7%) myelodysplastic syndromes (MDS), 16(29.6%) myelodysplastic/myeloproliferative neoplasms overlap (MDS/MPN), 9 (16.6%) myeloproliferative neoplasms (MPN) and 14 (25.9%) acute myeloid leukemia (AML). The median age was 67 years, 67% were males (Table 1).
A) GATA2 mutations characteristics and co-mutations: Median GATA2 mutation VAF was 31% in the total cohort (range 5-74%), with 28%, 37%, 34%, and 39% (p= .6) seen in MDS, MDS/MPN, MPN, and AML, respectively. 31 (57%) mutations clustered in the zinc finger 2 domain (ZF2), 9 (17%) in the ZF1 domain and 14 (26%) were outside the ZF domains (non-ZF). ZF1-mutated pts had lower mean VAF compared to ZF2 pts (22% vs. 38%, p=.03), and the majority occurred in AML cases (6/9, 67%), with the most common mutation type being missense (78%). There was a significant association between mutation type and location (p <.0001). The median number of mutations per pts was 4. We identified 28 co-mutations in 52 (96%) pts. ASXL1 (60%), SRSF2 (33%) and RUNX1 (19%) were the most common co-mutations. ASXL1 was frequently co-mutated with GATA2-ZF2 (74%) and non-ZF (22.6%) (only 1 co-mutated with GATA2-ZF1). ASXL1 was also more commonly co-mutated in chronic MN compared to AML (94% vs. 6%, p=.0001). KIT, CEBPA and PTPN11 were co-mutated only in ZF1-mutated pts. KIT, FLT3, PTPN11, CEBPA were co-mutated only in AML, whereas CALR was co-mutated only in MPN.
B) Survival outcome: After a median follow-up time of 26.4 months, 34 (63%) pts died with a median OS of 23.9, 22.4, 20.6, and 5.6 months in MDS, MDS/MPN, MPN, and AML pts; respectively. After excluding AML pts for survival analysis, we found that pts with several GATA2 mutations had poorer median OS (4.9 vs. 24.5 months, p=0.03) compared to those with single GATA2 mutation. MDS pts with chromatin modification co-mutations (BCOR, EZH2, ASXL1, KDM6A) (17 vs. 30 months, p=.04) or frameshift (FS) mutation (10.3 vs. 27.3 months, p=.05) had worse median OS compared to those without chromatin modification co-mutation or non-FS mutation, respectively. MDS/MPN overlap pts with RUNX1 co-mutation had worse median OS (2.2 vs. 25.7 months, p=.0004) than their un-mutated counterparts. There was no difference in median OS between GATA2-ZF1, ZF2, and non-ZF (30 vs. 20.6 vs. 22.4 months, p= .7).

Conclusion
We describe the spectrum and characteristics of GATA2 mutations across myeloid neoplasms. There was an impact of different GATA2-mutation types, single vs several GATA2 mutations, and co-mutations pattern on survival. Our work was limited by the small number of patients and inability to completely rule out germline mutation possibility.
Keyword(s): GATA-2, Myeloid malignancies, Outcome


