
Contributions
Abstract: S158
Type: Oral Presentation
Session title: Population based studies in myeloid disorders
Background
Ruxolitinib (RUXO) is a JAK1/2 inhibitor approved for the treatment of patients (pts) with primary (PMF) and secondary myelofibrosis (sMF). In randomized controlled trials, RUXO was effective in controlling symptoms and splenomegaly, while survival improvement, reported in post-hoc analysis, is still debated. In 2013 the ERNEST registry was activated to prospectively enroll MF patients receiving ruxolitinib.
Aims
The aim of the ERNEST project was to collect estimates of clinical outcome of contemporary MF pts in real-life settings.
Methods
Current study describes updates with cut-off deadline at dec 31, 2018 of those ERNEST patients who were reported already as alive and/or in active surveillance at Nov 2014 (Blood 2014; 124:1849). Italy, Spain and Sweden participated in the Registry update. The chi-square test or Fisher’s exact test (categorical variables) and t-test or Wilcoxon-Mann-Whitney test (continuous variables) were used. Kaplan-Meier analysis was used for OS. To avoid immortal time bias, only pts exposed to cytoreductive therapy were included and followed from the start of treatment until death or censoring (last contact/study-end fixed at Dec 31, 2018), whichever came first. A multivariable Cox proportional-hazard model was used to investigate predictors of mortality. To assure comparability between patients treated with Hydroxyurea (pts-HU) and RUXO (pts-RUXO), we conducted a propensity score (PS) matching analysis. The PS included age at first administration, gender, type of MF, DIPSS at first administration, year of diagnosis, palpable spleen at first administration and time to first administration. 2‐tailed p-values <0.05 were considered significant.
Results
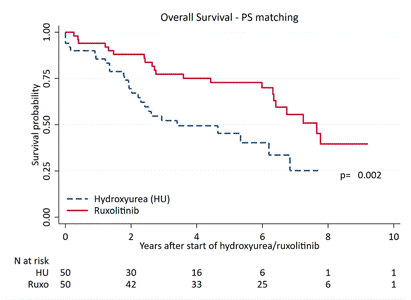
At cut-off, the data of 1,010 pts were analyzed; there were 68% PMF, 20% PET-MF, 22% PPV-MF (median age at diagnosis: 64y). 23%,37% and 40% of diagnosis were performed between 2001-2004, 2005-2008 and 2009-2012. After a median follow-up of 5.2y (2.3–8.2), 625 deaths occurred with a mortality rate (per 100 person-years) of 10.9 (95%CI 10.1-11.8). Median OS: 6.2y (95%CI 2.8-12.6), with no significant difference according to diagnostic categories (P=0.4). Among pts treated with cytoreduction during follow-up (59.2% of total), at first administration pts-RUXO were significantly younger (64.yrs vs 67.0 yrs, p=0.02), with massive splenomegaly (>20cm from LCM; 37% vs 6.0%, p<0.001) and higher incidence of constitutional symptoms (80% vs 49%;p=0.03) compared to pts-HU. Time to first treatment with HU (median time 0.0y, range 0-1.2y) was significantly shorter than RUXO (median 4.5y range 2.2-6.7y; p<0.001); 64% of pts-RUXO had been previously treated with HU, in 2 cases with interferon. OS was significantly longer in pts-RUXO compared to pts-HU (6.7y vs 5.1y; P=0.001); for those in higher DIPSS category OS was 6.4y for pts-RUXO vs 3.0 for pts-HU (p=0.003). Multivariable analysis identified the following factors that negatively affected OS: age (linear covariate, HR 1.03,p<0.001), male gender (HR 1.58, p<0.001), high DIPSS category (HR 2.96,p<0.001). Conversely, protective variables were more recent diagnosis (2009-2012 vs 2001-2004; HR 0.47,p<0.01) and exposure to ruxolitinib (HR 0.62,p=0.029). After PS matching (n=50 in each group), median OS was 7.7y in pts-RUXO vs 3.4y in pts-HU, respectively (p=0.002;Fig.1), and was not statistically different in those who used RUXO as first line or after HU (6.4y vs 7.8y, p=1.00).
Conclusion
Within the limitations of an observational study, these real-life data support an overall survival benefit of RUXO over HU alone in pts with PMF and sMF
Keyword(s): Hydroxyurea, Janus Kinase inhibitor, Myelofibrosis, Survival prediction
Abstract: S158
Type: Oral Presentation
Session title: Population based studies in myeloid disorders
Background
Ruxolitinib (RUXO) is a JAK1/2 inhibitor approved for the treatment of patients (pts) with primary (PMF) and secondary myelofibrosis (sMF). In randomized controlled trials, RUXO was effective in controlling symptoms and splenomegaly, while survival improvement, reported in post-hoc analysis, is still debated. In 2013 the ERNEST registry was activated to prospectively enroll MF patients receiving ruxolitinib.
Aims
The aim of the ERNEST project was to collect estimates of clinical outcome of contemporary MF pts in real-life settings.
Methods
Current study describes updates with cut-off deadline at dec 31, 2018 of those ERNEST patients who were reported already as alive and/or in active surveillance at Nov 2014 (Blood 2014; 124:1849). Italy, Spain and Sweden participated in the Registry update. The chi-square test or Fisher’s exact test (categorical variables) and t-test or Wilcoxon-Mann-Whitney test (continuous variables) were used. Kaplan-Meier analysis was used for OS. To avoid immortal time bias, only pts exposed to cytoreductive therapy were included and followed from the start of treatment until death or censoring (last contact/study-end fixed at Dec 31, 2018), whichever came first. A multivariable Cox proportional-hazard model was used to investigate predictors of mortality. To assure comparability between patients treated with Hydroxyurea (pts-HU) and RUXO (pts-RUXO), we conducted a propensity score (PS) matching analysis. The PS included age at first administration, gender, type of MF, DIPSS at first administration, year of diagnosis, palpable spleen at first administration and time to first administration. 2‐tailed p-values <0.05 were considered significant.
Results
At cut-off, the data of 1,010 pts were analyzed; there were 68% PMF, 20% PET-MF, 22% PPV-MF (median age at diagnosis: 64y). 23%,37% and 40% of diagnosis were performed between 2001-2004, 2005-2008 and 2009-2012. After a median follow-up of 5.2y (2.3–8.2), 625 deaths occurred with a mortality rate (per 100 person-years) of 10.9 (95%CI 10.1-11.8). Median OS: 6.2y (95%CI 2.8-12.6), with no significant difference according to diagnostic categories (P=0.4). Among pts treated with cytoreduction during follow-up (59.2% of total), at first administration pts-RUXO were significantly younger (64.yrs vs 67.0 yrs, p=0.02), with massive splenomegaly (>20cm from LCM; 37% vs 6.0%, p<0.001) and higher incidence of constitutional symptoms (80% vs 49%;p=0.03) compared to pts-HU. Time to first treatment with HU (median time 0.0y, range 0-1.2y) was significantly shorter than RUXO (median 4.5y range 2.2-6.7y; p<0.001); 64% of pts-RUXO had been previously treated with HU, in 2 cases with interferon. OS was significantly longer in pts-RUXO compared to pts-HU (6.7y vs 5.1y; P=0.001); for those in higher DIPSS category OS was 6.4y for pts-RUXO vs 3.0 for pts-HU (p=0.003). Multivariable analysis identified the following factors that negatively affected OS: age (linear covariate, HR 1.03,p<0.001), male gender (HR 1.58, p<0.001), high DIPSS category (HR 2.96,p<0.001). Conversely, protective variables were more recent diagnosis (2009-2012 vs 2001-2004; HR 0.47,p<0.01) and exposure to ruxolitinib (HR 0.62,p=0.029). After PS matching (n=50 in each group), median OS was 7.7y in pts-RUXO vs 3.4y in pts-HU, respectively (p=0.002;Fig.1), and was not statistically different in those who used RUXO as first line or after HU (6.4y vs 7.8y, p=1.00).

Conclusion
Within the limitations of an observational study, these real-life data support an overall survival benefit of RUXO over HU alone in pts with PMF and sMF
Keyword(s): Hydroxyurea, Janus Kinase inhibitor, Myelofibrosis, Survival prediction


