
Contributions
Abstract: PB1826
Type: Publication Only
Session title: Thrombosis and vascular biology - Biology & Translational Research
Background
A biosimilar is a medicine of biotechnological origin, produced according to specific requirements established by the European Medicines Agency (EMA) regarding quality, efficacy and safety.
Aims
The main objective is to evaluate the efficacy and safety of using enoxaparin sodium biosimilar (Inhixa ®) available in our center, for the prophylaxis of venous thromboembolic disease and the treatment of deep vein thrombosis, as well as hemorrhagic or thrombotic complications.
Methods
We evaluated two groups of patients with enoxaparin sodium biosimilar:
First group: Patients continuously anticoagulated at full doses, who were suspended oral anticoagulation due to recurrent hemorrhagic diathesis, active cancer under chemotherapy treatment and / or thrombosis.
- 25 patients treated with heparin at weight adapted doses once a day, as a starting dose, and then subsequently, according to anti-Xa levels.
- Ages between 50 and 88 years old.
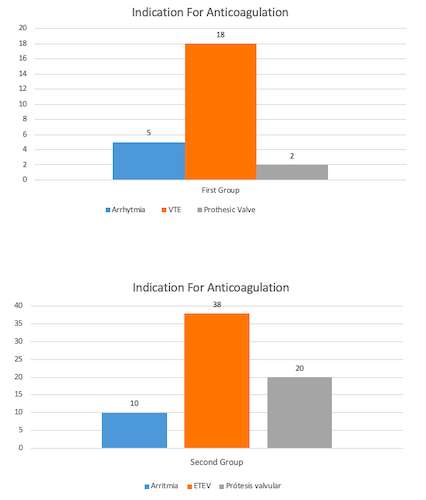
- Indication of main anticoagulation: 5 arrhythmia, 18 VTE and 2 prosthetic valves.
- Most of the cases presented several associated diagnoses, and 75% of them comorbidities.
Second group: Anticoagulated patients with VKA with high thromboembolic risk, who have required bridging therapy at full doses.
- 98 patients. Bridging therapy consisted of suspending VKA from 4 (acenocoumarol) to 6 days (warfarin) before the procedure and substitution with biosimilar enoxaparin sodium: 1.5 mg / kg / 24 h, and administration of a prophylactic dose 4,000 IU, 12 hours before of the procedure, and another dose 6-12 hours after it, depending on the risk of bleeding from the intervention and the thrombotic risk of the patient's underlying disease.
- The ages ranged from 21 to 87 years.
- Indication for main anticoagulation: 10 arrhythmias, 58 VTE (with or without thrombophilia) and 30 prosthetic valves.
Results
- In patients who had secondary bleeding to VKA, it totally disappeared with heparin.
- As side effects, only 3 patients had complications, being allergic reaction to heparin.
- There were no bleeding or thrombotic phenomena.
- There were 6 cases of hematomas at the heparin puncture sites.
Conclusion
Although these are few cases, the results obtained confirm the efficacy, safety and cost-effectiveness of the continuous use of enoxaparin sodium biosimilar in the prevention of VTE in patients requiring anticoagulation, when VKA are contraindicated and and DOACs cannot be managed. Anti-Xa levels are very useful for dose adjustment when necessary.
Biosimilar enoxaparin sodium administered at therapeutic doses in the perioperative period, according to the scheme described, is associated with a low incidence of VTE recurrence and bleeding. Treatment should be personalized according to each patient and factors related to the surgical intervention.
Further studies will provide more confirmatory data. Biosimilar drugs have the potential to offer the National Health System cost savings and expand access to therapeutic innovations.
Keyword(s): Enoxaparin, Hemorrhage, Heparin, Thrombosis
Abstract: PB1826
Type: Publication Only
Session title: Thrombosis and vascular biology - Biology & Translational Research
Background
A biosimilar is a medicine of biotechnological origin, produced according to specific requirements established by the European Medicines Agency (EMA) regarding quality, efficacy and safety.
Aims
The main objective is to evaluate the efficacy and safety of using enoxaparin sodium biosimilar (Inhixa ®) available in our center, for the prophylaxis of venous thromboembolic disease and the treatment of deep vein thrombosis, as well as hemorrhagic or thrombotic complications.
Methods
We evaluated two groups of patients with enoxaparin sodium biosimilar:
First group: Patients continuously anticoagulated at full doses, who were suspended oral anticoagulation due to recurrent hemorrhagic diathesis, active cancer under chemotherapy treatment and / or thrombosis.
- 25 patients treated with heparin at weight adapted doses once a day, as a starting dose, and then subsequently, according to anti-Xa levels.
- Ages between 50 and 88 years old.
- Indication of main anticoagulation: 5 arrhythmia, 18 VTE and 2 prosthetic valves.
- Most of the cases presented several associated diagnoses, and 75% of them comorbidities.
Second group: Anticoagulated patients with VKA with high thromboembolic risk, who have required bridging therapy at full doses.
- 98 patients. Bridging therapy consisted of suspending VKA from 4 (acenocoumarol) to 6 days (warfarin) before the procedure and substitution with biosimilar enoxaparin sodium: 1.5 mg / kg / 24 h, and administration of a prophylactic dose 4,000 IU, 12 hours before of the procedure, and another dose 6-12 hours after it, depending on the risk of bleeding from the intervention and the thrombotic risk of the patient's underlying disease.
- The ages ranged from 21 to 87 years.
- Indication for main anticoagulation: 10 arrhythmias, 58 VTE (with or without thrombophilia) and 30 prosthetic valves.
Results
- In patients who had secondary bleeding to VKA, it totally disappeared with heparin.
- As side effects, only 3 patients had complications, being allergic reaction to heparin.
- There were no bleeding or thrombotic phenomena.
- There were 6 cases of hematomas at the heparin puncture sites.

Conclusion
Although these are few cases, the results obtained confirm the efficacy, safety and cost-effectiveness of the continuous use of enoxaparin sodium biosimilar in the prevention of VTE in patients requiring anticoagulation, when VKA are contraindicated and and DOACs cannot be managed. Anti-Xa levels are very useful for dose adjustment when necessary.
Biosimilar enoxaparin sodium administered at therapeutic doses in the perioperative period, according to the scheme described, is associated with a low incidence of VTE recurrence and bleeding. Treatment should be personalized according to each patient and factors related to the surgical intervention.
Further studies will provide more confirmatory data. Biosimilar drugs have the potential to offer the National Health System cost savings and expand access to therapeutic innovations.
Keyword(s): Enoxaparin, Hemorrhage, Heparin, Thrombosis


