
Contributions
Abstract: PB1810
Type: Publication Only
Session title: Thalassemias
Background
Hydroxyurea (HU) has been widely used in clinical practice to manage patients with non-transfusion dependent thalassaemia (NTDT). Few data are available about the effects of its administration in Italian patients.
Aims
This multicenter study aimed to assess haematological and non-haematological outcomes following short- and long-term exposure to HU.
Methods
Among the 79 NTDT patients (<4 transfusions per year) consecutively enrolled in the Myocardial Iron Overload in Thalassaemia (MIOT) network, we considered 71 patients (30 females) treated for >12 months with HU. Patients were followed prospectively until September 2019.
Results
Patients started the treatment with HU at a mean age of 37.0±12.1 years and anemia was the indication in the 88.6% of the cases. The mean duration of HU treatment was 8.2±5.8 years.
After 12 months of therapy a significant increase in hemoglobin and mean corpuscular volume values and a significant decrease in the number of white blood cells, indirect bilirubin, nucleated red blood cells, and platelets, in uric acid levels, and in soluble transferrin receptor values were detected. We defined responders the 28 patients who achieved a ≥1.0 g/dl increase in haemoglobin levels after 12 months. Responders received a significantly higher dose of HU and showed significantly lower pre-treatment haemoglobin values. At receiving operating curve analysis, a dosage greater than 16.6 mg/dl discriminated the responders with acceptable sensitivity and good specificity (59.3% and 85.7%, respectively) and with an area under the curve of 0.75 (P<0.0001). The responders experienced a significantly higher decrease in white blood cells after 12 months of treatment.
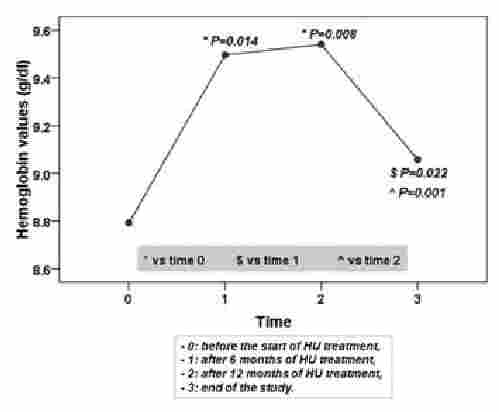
The hematological response dropped in long-term treatment (see Figure).
During the HU treatment we did not detect significant changes in the frequency of dyspnea, asthenia, atrial dilatations, cardiovascular complications (pulmonary hypertension, arrhythmias, ulcers, and deep vein thrombosis), extramedullary hematopotesis (EMH), and fibroadipose involution of the EMH masses.
Conclusion
This study seems to suggest that not only for the hematological responder patients, but also for those affected by specific complications, HU could be still a valid option to limit the advance of the overall clinical burden disease without carrying significant adverse events and increase in mortality.
Keyword(s): Hydroxyurea, Thalassemia
Abstract: PB1810
Type: Publication Only
Session title: Thalassemias
Background
Hydroxyurea (HU) has been widely used in clinical practice to manage patients with non-transfusion dependent thalassaemia (NTDT). Few data are available about the effects of its administration in Italian patients.
Aims
This multicenter study aimed to assess haematological and non-haematological outcomes following short- and long-term exposure to HU.
Methods
Among the 79 NTDT patients (<4 transfusions per year) consecutively enrolled in the Myocardial Iron Overload in Thalassaemia (MIOT) network, we considered 71 patients (30 females) treated for >12 months with HU. Patients were followed prospectively until September 2019.
Results
Patients started the treatment with HU at a mean age of 37.0±12.1 years and anemia was the indication in the 88.6% of the cases. The mean duration of HU treatment was 8.2±5.8 years.
After 12 months of therapy a significant increase in hemoglobin and mean corpuscular volume values and a significant decrease in the number of white blood cells, indirect bilirubin, nucleated red blood cells, and platelets, in uric acid levels, and in soluble transferrin receptor values were detected. We defined responders the 28 patients who achieved a ≥1.0 g/dl increase in haemoglobin levels after 12 months. Responders received a significantly higher dose of HU and showed significantly lower pre-treatment haemoglobin values. At receiving operating curve analysis, a dosage greater than 16.6 mg/dl discriminated the responders with acceptable sensitivity and good specificity (59.3% and 85.7%, respectively) and with an area under the curve of 0.75 (P<0.0001). The responders experienced a significantly higher decrease in white blood cells after 12 months of treatment.
The hematological response dropped in long-term treatment (see Figure).
During the HU treatment we did not detect significant changes in the frequency of dyspnea, asthenia, atrial dilatations, cardiovascular complications (pulmonary hypertension, arrhythmias, ulcers, and deep vein thrombosis), extramedullary hematopotesis (EMH), and fibroadipose involution of the EMH masses.

Conclusion
This study seems to suggest that not only for the hematological responder patients, but also for those affected by specific complications, HU could be still a valid option to limit the advance of the overall clinical burden disease without carrying significant adverse events and increase in mortality.
Keyword(s): Hydroxyurea, Thalassemia


