Contributions
Abstract: PB1809
Type: Publication Only
Session title: Thalassemias
Background
Growth retardation (GR) and pubertal disorders are the earliest consequences of iron toxicity resulting from the pituitary iron deposition in children and adolescents with thalassemia major (TM). It is suggested that appropriate transfusion and iron chelation therapy (ICT) support proper growth and development.
Aims
The purpose of this study was to evaluate if the current treatment protocols may support a normal growth progression and sexual development in patients (pts) with TM.
Methods
We recruited male (M) and female (F) TM pts born after 2000. The pts with an acute or chronic illness that may interfere with growth and development or stem cell transplantation recipients were excluded. The Ethical Committee of Clinical Investigation (19.02.2020, 20-2.1T/2) approved this study. Transfused packed red cell units (pRBC) and average pre-transfusion hemoglobin (Hb) per year were recorded. Serum ferritin (SF) was monitored monthly (m). Myocardial (T2*) and liver (R2) iron were assessed by magnetic resonance imaging (MRI) after 8-years (y) of age. Annual median SF, prescribed chelator(s), mean chelator doses were recorded. Transfusional iron intake (TII) was estimated and expressed as mg/ kg b.w./days. Height (H) and weight (W) assessments were performed using the Harpenden stadiometer, and puberty was evaluated by Tanner staging at 3 monthly. H-standard deviation scores (h-SDS) were calculated. All pts were assessed annually for bone mineral density (BMD) after 8 y. The mean SDS of the lumbar spine (L1-4) BMD between -1 and -2.5 was defined as osteopenia and <-2.5 as osteoporosis. Growth hormone (GH) secretion was assessed by L-dopa and insulin tests in those with standing-H below the –2 SD. Pts whose GH response to both stimulation tests (STs) was less than 5 ng/ml with the monoclonal assay was defined as GH deficiency (GHD).
Results
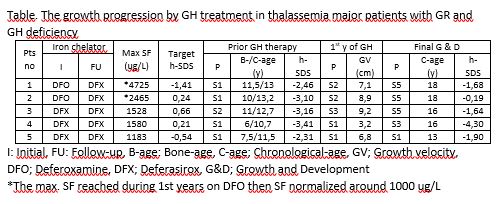
We evaluated 30 TM (28 β/β, 1 β/δβ, 1 HbH) pts (13 F and 17 M) with the mean age of 12,5 ± 4,5 y. All pts have received pRBC for 11,1 ± 4,4 y and maintained pre-transfusion Hb> 9 g/dl. The patients started ICT by the SF of 1430 ± 795 ug/L and maintained ≤1500 ug/L at all times during 9 ± 4.2 y. Twenty-four pts received only Deferasirox (DFX) chelation while 6 were initiated ICT by Deferoxamine (DFO) and continued with DFX. Liver iron concentration (LIC) and myocardial T2* (mT2*) in 22 pts were between 0.9-5.4 (2.1 ± 1.3 SD) mg Fe/g d.w. and 19.4-43.5 (28 ± 6.7 SD) ms, respectively, in all assessments. The puberty initiated at 12.8 ± 1.1 y in M (n=12) & 11.2 ± 1.3 y in F (n=12) and progressed without hormone replacement in all. Overall, growth velocity reduced compared to the non-thalassemic population after 8 y with clear evidence of growth catch-up by the onset of puberty. Five pts (16.5%) developed h-SDS< -2 at 10-12 y-old when all but one had S1/2 puberty and displayed GHD. All pts received GH therapy. The pt no 4 was entirely unresponsive to GH-STs, developed GR at 1 y-old, and has remained unresponsive to GH therapy (Table). The 14 (%58) and 5 (21%) of 24 pts demonstrated osteopenia and osteoporosis, respectively. The BMD-SDS improved following pubertal spurt.
Conclusion
The high prevalence of GHD in TM patients receiving an optimum transfusion and ICT throughout their life was disappointed. The most probable explanation is, growth patterns and bone-maturation in TM pts might differ from the non-thalassemic population, and the GH-STs may lead to overdiagnosis of GH deficiency in this distinct population.
Keyword(s): Growth hormone, Iron chelation, Iron overload, Thalassemia
Abstract: PB1809
Type: Publication Only
Session title: Thalassemias
Background
Growth retardation (GR) and pubertal disorders are the earliest consequences of iron toxicity resulting from the pituitary iron deposition in children and adolescents with thalassemia major (TM). It is suggested that appropriate transfusion and iron chelation therapy (ICT) support proper growth and development.
Aims
The purpose of this study was to evaluate if the current treatment protocols may support a normal growth progression and sexual development in patients (pts) with TM.
Methods
We recruited male (M) and female (F) TM pts born after 2000. The pts with an acute or chronic illness that may interfere with growth and development or stem cell transplantation recipients were excluded. The Ethical Committee of Clinical Investigation (19.02.2020, 20-2.1T/2) approved this study. Transfused packed red cell units (pRBC) and average pre-transfusion hemoglobin (Hb) per year were recorded. Serum ferritin (SF) was monitored monthly (m). Myocardial (T2*) and liver (R2) iron were assessed by magnetic resonance imaging (MRI) after 8-years (y) of age. Annual median SF, prescribed chelator(s), mean chelator doses were recorded. Transfusional iron intake (TII) was estimated and expressed as mg/ kg b.w./days. Height (H) and weight (W) assessments were performed using the Harpenden stadiometer, and puberty was evaluated by Tanner staging at 3 monthly. H-standard deviation scores (h-SDS) were calculated. All pts were assessed annually for bone mineral density (BMD) after 8 y. The mean SDS of the lumbar spine (L1-4) BMD between -1 and -2.5 was defined as osteopenia and <-2.5 as osteoporosis. Growth hormone (GH) secretion was assessed by L-dopa and insulin tests in those with standing-H below the –2 SD. Pts whose GH response to both stimulation tests (STs) was less than 5 ng/ml with the monoclonal assay was defined as GH deficiency (GHD).
Results
We evaluated 30 TM (28 β/β, 1 β/δβ, 1 HbH) pts (13 F and 17 M) with the mean age of 12,5 ± 4,5 y. All pts have received pRBC for 11,1 ± 4,4 y and maintained pre-transfusion Hb> 9 g/dl. The patients started ICT by the SF of 1430 ± 795 ug/L and maintained ≤1500 ug/L at all times during 9 ± 4.2 y. Twenty-four pts received only Deferasirox (DFX) chelation while 6 were initiated ICT by Deferoxamine (DFO) and continued with DFX. Liver iron concentration (LIC) and myocardial T2* (mT2*) in 22 pts were between 0.9-5.4 (2.1 ± 1.3 SD) mg Fe/g d.w. and 19.4-43.5 (28 ± 6.7 SD) ms, respectively, in all assessments. The puberty initiated at 12.8 ± 1.1 y in M (n=12) & 11.2 ± 1.3 y in F (n=12) and progressed without hormone replacement in all. Overall, growth velocity reduced compared to the non-thalassemic population after 8 y with clear evidence of growth catch-up by the onset of puberty. Five pts (16.5%) developed h-SDS< -2 at 10-12 y-old when all but one had S1/2 puberty and displayed GHD. All pts received GH therapy. The pt no 4 was entirely unresponsive to GH-STs, developed GR at 1 y-old, and has remained unresponsive to GH therapy (Table). The 14 (%58) and 5 (21%) of 24 pts demonstrated osteopenia and osteoporosis, respectively. The BMD-SDS improved following pubertal spurt.
Conclusion
The high prevalence of GHD in TM patients receiving an optimum transfusion and ICT throughout their life was disappointed. The most probable explanation is, growth patterns and bone-maturation in TM pts might differ from the non-thalassemic population, and the GH-STs may lead to overdiagnosis of GH deficiency in this distinct population.
Keyword(s): Growth hormone, Iron chelation, Iron overload, Thalassemia