
Contributions
Abstract: PB1808
Type: Publication Only
Session title: Thalassemias
Background
Beta-thalassemia is characterized by ineffective erythropoiesis, leading to a decreased number of mature and functional RBC, resulting in mild to severe anemia. Luspatercept is the first approved disease-modifying drug, currently indicated for Transfusion-Dependent Thalassemia (TDT) and in advanced trials for Non Transfusion-Dependent Thalassemia (NTDT). The response to luspatercept is quantified by the increase in total hemoglobin (Hb) in NTDT and by the decrease in transfusional need for TDT, but both changes require a long time to be consistently assessed.
Aims
To explore the hypothesis that early modifications in red blood cell indices could have a predictive value of response to luspatercept.
Methods
As reference Centre for Hemoglobinopathies of University of Torino we took part of luspatercept phase II/III clinical trials (NCT01749540, NCT02268409, NCT02604433, NCT03342404, NCT04064060). In this analysis we considered only local blood tests for all our patients that received luspatercept; patients or time frames on placebo were excluded. Responders and non responders were defined according to published clinical trial protocols: specifically, an increase in Hb ≥ 1.5 g/dL (NTDT) or a reduction in RBC transfusion need ≥ 33% (TDT) from baseline in weeks 13 to 24.
Results
The overall time of luspatercept treatment has been 32.0 years. The median duration for NTDT was 2.4 years (range 0.2-6.0) and for TDT 1.7 years (range 0.3-6.0) for each patient. We reviewed 1024 blood tests of 14 NTDT patients (9 responders, 5 non-responders) and 1968 blood tests of 14 TDT patients (10 responders and 4 non-responders).
No predictor of response was identified in blood test indices of TDT patients.
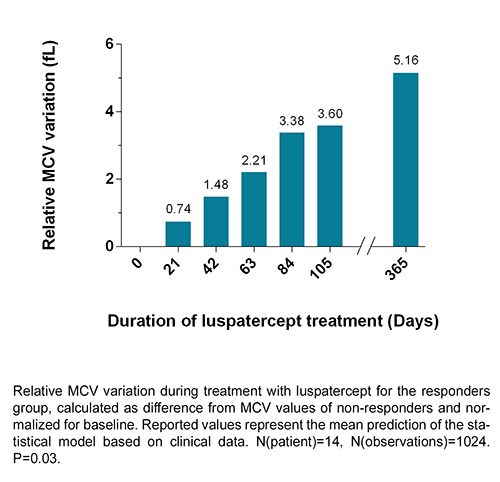
In NTDT patients, beyond the hemoglobin that defines the response, the index with better discriminative power was the MCV. A statistically significant trend difference in MCV was observed between responders and non-responders (p=0.03). Although the difference between groups is significant from 27 weeks after treatment start, responders show a relative increase in MCV compared to non-responders from the first weeks of treatment, reaching a mean variation of +5.16 fL at one year (see figure). The change remains long term. At baseline responders and non responders did not differ for age, genotype and total Hb, whereas mean baseline MCV was lower in responders (66.1 vs 72.3 fL), but this difference was not significant.
Conclusion
NTDT patients who respond to luspatercept treatment show an early increase in MCV; this change keeps up to 6 years. In part, this could be due to the rise in reticulocytes. In our data, a variation of reticulocytes alone is not sufficient to predict the response, but this could be due to the small sample size. More likely the MCV improvement could be the result of the not fully understood effects of luspatercept in regulating the late-stage RBC maturation.
These data need verification on a large set. If confirmed, the early MCV change may be easily applied in the clinical setting to predict long-term response to luspatercept among NTDT patients.
Keyword(s): Beta thalassemia, Drug sensitivity, Erythropoieisis
Abstract: PB1808
Type: Publication Only
Session title: Thalassemias
Background
Beta-thalassemia is characterized by ineffective erythropoiesis, leading to a decreased number of mature and functional RBC, resulting in mild to severe anemia. Luspatercept is the first approved disease-modifying drug, currently indicated for Transfusion-Dependent Thalassemia (TDT) and in advanced trials for Non Transfusion-Dependent Thalassemia (NTDT). The response to luspatercept is quantified by the increase in total hemoglobin (Hb) in NTDT and by the decrease in transfusional need for TDT, but both changes require a long time to be consistently assessed.
Aims
To explore the hypothesis that early modifications in red blood cell indices could have a predictive value of response to luspatercept.
Methods
As reference Centre for Hemoglobinopathies of University of Torino we took part of luspatercept phase II/III clinical trials (NCT01749540, NCT02268409, NCT02604433, NCT03342404, NCT04064060). In this analysis we considered only local blood tests for all our patients that received luspatercept; patients or time frames on placebo were excluded. Responders and non responders were defined according to published clinical trial protocols: specifically, an increase in Hb ≥ 1.5 g/dL (NTDT) or a reduction in RBC transfusion need ≥ 33% (TDT) from baseline in weeks 13 to 24.
Results
The overall time of luspatercept treatment has been 32.0 years. The median duration for NTDT was 2.4 years (range 0.2-6.0) and for TDT 1.7 years (range 0.3-6.0) for each patient. We reviewed 1024 blood tests of 14 NTDT patients (9 responders, 5 non-responders) and 1968 blood tests of 14 TDT patients (10 responders and 4 non-responders).
No predictor of response was identified in blood test indices of TDT patients.
In NTDT patients, beyond the hemoglobin that defines the response, the index with better discriminative power was the MCV. A statistically significant trend difference in MCV was observed between responders and non-responders (p=0.03). Although the difference between groups is significant from 27 weeks after treatment start, responders show a relative increase in MCV compared to non-responders from the first weeks of treatment, reaching a mean variation of +5.16 fL at one year (see figure). The change remains long term. At baseline responders and non responders did not differ for age, genotype and total Hb, whereas mean baseline MCV was lower in responders (66.1 vs 72.3 fL), but this difference was not significant.

Conclusion
NTDT patients who respond to luspatercept treatment show an early increase in MCV; this change keeps up to 6 years. In part, this could be due to the rise in reticulocytes. In our data, a variation of reticulocytes alone is not sufficient to predict the response, but this could be due to the small sample size. More likely the MCV improvement could be the result of the not fully understood effects of luspatercept in regulating the late-stage RBC maturation.
These data need verification on a large set. If confirmed, the early MCV change may be easily applied in the clinical setting to predict long-term response to luspatercept among NTDT patients.
Keyword(s): Beta thalassemia, Drug sensitivity, Erythropoieisis


