
Contributions
Abstract: PB1805
Type: Publication Only
Session title: Thalassemias
Background
Thalassemias are characterized by imbalanced globin-chain production resulting in excess α- or β-globin precipitation, hemolytic anemia, and ineffective erythropoiesis. Adenosine triphosphate (ATP) levels are reduced in thalassemic red blood cells (RBCs), despite increased energy demands. Mitapivat (AG-348) is an oral activator of RBC pyruvate kinase (PKR), a glycolytic enzyme that regulates ATP production. In a phase 2 study of patients (pts) with α- or β-non–transfusion-dependent thalassemia (NTDT), twice-daily (BID) dosing with mitapivat increased hemoglobin (Hb) levels by ≥1.0 g/dL in 80% of pts, supporting the broadening of mitapivat’s development in thalassemia.
Aims
To report the study designs of ENERGIZE (EudraCT: 2021-000211-23) and ENERGIZE-T (EudraCT: 2021-000212-34), two phase 3 trials to assess the efficacy and safety of mitapivat in adults with α- or β-NTDT or transfusion-dependent thalassemia (TDT), respectively.
Methods
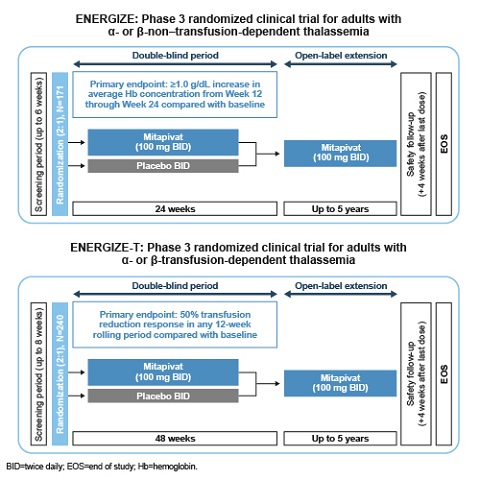
Both studies are phase 3, multicenter, randomized, double-blind, placebo-controlled trials. In ENERGIZE, approximately 171 eligible adults with NTDT will be randomized (2:1) to receive 100 mg mitapivat BID or placebo for 24 weeks (wks). Upon completion, eligible pts can transition to a 5-year, open-label extension. Key inclusion criteria: documented diagnosis of thalassemia (β-thalassemia ± α-globin mutations, Hb E β-thalassemia, or α-thalassemia [Hb H disease]), Hb concentration ≤10.0 g/dL, and NTDT defined as ≤5 RBC units during the 24-wk period before randomization and no RBC transfusion ≤8 wks prior. The primary endpoint is an Hb response defined as a ≥1.0 g/dL increase in average Hb concentration from Wk 12 through 24 compared with baseline (BL). Secondary endpoints include pt-reported outcomes, changes in Hb, markers of hemolysis and erythropoiesis, and safety. In ENERGIZE-T, approximately 240 eligible adults with TDT will be randomized (2:1) to receive 100 mg mitapivat BID or placebo for 48 wks. Upon completion, eligible pts can transition to a 5-year, open-label extension. Key inclusion criteria: documented diagnosis of thalassemia (same genotypes as detailed for the ENERGIZE study), and TDT defined as 6–20 RBC units transfused and no transfusion-free period ≥6 wks during the 24 wks before randomization. The primary endpoint is a transfusion reduction response, defined as a ≥50% reduction in transfused RBC units with a reduction of ≥2 units of transfused RBCs in any consecutive 12-wk period through Wk 48 compared with BL. Secondary endpoints include additional measures of transfusion burden, changes in iron markers, and safety.
Results
Not yet available.
Conclusion
ENERGIZE and ENERGIZE-T are the first pivotal studies to assess a potential treatment across a broad spectrum of pts with thalassemia (ie, pts with TDT and NTDT; α- and β-thalassemias). This phase 3 program will evaluate the efficacy and safety of mitapivat, a novel, first-in-class oral activator of PKR. Both studies will start enrollment in 2021.
Keyword(s): AG-348, Hemolytic anemia, Pyruvate kinase, Thalassemia
Abstract: PB1805
Type: Publication Only
Session title: Thalassemias
Background
Thalassemias are characterized by imbalanced globin-chain production resulting in excess α- or β-globin precipitation, hemolytic anemia, and ineffective erythropoiesis. Adenosine triphosphate (ATP) levels are reduced in thalassemic red blood cells (RBCs), despite increased energy demands. Mitapivat (AG-348) is an oral activator of RBC pyruvate kinase (PKR), a glycolytic enzyme that regulates ATP production. In a phase 2 study of patients (pts) with α- or β-non–transfusion-dependent thalassemia (NTDT), twice-daily (BID) dosing with mitapivat increased hemoglobin (Hb) levels by ≥1.0 g/dL in 80% of pts, supporting the broadening of mitapivat’s development in thalassemia.
Aims
To report the study designs of ENERGIZE (EudraCT: 2021-000211-23) and ENERGIZE-T (EudraCT: 2021-000212-34), two phase 3 trials to assess the efficacy and safety of mitapivat in adults with α- or β-NTDT or transfusion-dependent thalassemia (TDT), respectively.
Methods
Both studies are phase 3, multicenter, randomized, double-blind, placebo-controlled trials. In ENERGIZE, approximately 171 eligible adults with NTDT will be randomized (2:1) to receive 100 mg mitapivat BID or placebo for 24 weeks (wks). Upon completion, eligible pts can transition to a 5-year, open-label extension. Key inclusion criteria: documented diagnosis of thalassemia (β-thalassemia ± α-globin mutations, Hb E β-thalassemia, or α-thalassemia [Hb H disease]), Hb concentration ≤10.0 g/dL, and NTDT defined as ≤5 RBC units during the 24-wk period before randomization and no RBC transfusion ≤8 wks prior. The primary endpoint is an Hb response defined as a ≥1.0 g/dL increase in average Hb concentration from Wk 12 through 24 compared with baseline (BL). Secondary endpoints include pt-reported outcomes, changes in Hb, markers of hemolysis and erythropoiesis, and safety. In ENERGIZE-T, approximately 240 eligible adults with TDT will be randomized (2:1) to receive 100 mg mitapivat BID or placebo for 48 wks. Upon completion, eligible pts can transition to a 5-year, open-label extension. Key inclusion criteria: documented diagnosis of thalassemia (same genotypes as detailed for the ENERGIZE study), and TDT defined as 6–20 RBC units transfused and no transfusion-free period ≥6 wks during the 24 wks before randomization. The primary endpoint is a transfusion reduction response, defined as a ≥50% reduction in transfused RBC units with a reduction of ≥2 units of transfused RBCs in any consecutive 12-wk period through Wk 48 compared with BL. Secondary endpoints include additional measures of transfusion burden, changes in iron markers, and safety.
Results
Not yet available.

Conclusion
ENERGIZE and ENERGIZE-T are the first pivotal studies to assess a potential treatment across a broad spectrum of pts with thalassemia (ie, pts with TDT and NTDT; α- and β-thalassemias). This phase 3 program will evaluate the efficacy and safety of mitapivat, a novel, first-in-class oral activator of PKR. Both studies will start enrollment in 2021.
Keyword(s): AG-348, Hemolytic anemia, Pyruvate kinase, Thalassemia


