
Contributions
Abstract: PB1790
Type: Publication Only
Session title: Stem cell transplantation - Clinical
Background
Steroid refractory (SR) and dependent (SD) acute or chronic Graft versus Host Disease (aGvHD/cGvHD) are severe complications of allogenic Hematopoietic Stem Cell Transplantation (HSCT) with high morbidity and mortality rates.
Extracorporeal Photopheresis (ECP) is a therapeutic option with T-cell immunoregulatory activity but without ImmunoSuppressive (IS) effects.
Aims
We retrospectively evaluated efficacy and safety of ECP in our pediatric population.
Methods
We reviewed data from 23 children with SR or SD GvHD followed at our Institution since 2010.
ECP was performed by COBE SpectraTMand Therakos CELLEXTM systems; the schedule consisted of 2 consecutive days each week for 4-12 weeks with tapering to biweekly/monthly regimens, adjusted by patient response.
Complete and partial responses (CR and PR) were defined by resolution of all signs and symptoms of GvHD in every organ and by 1 grade improvement in 1 organ without any progression, respectively.
Results
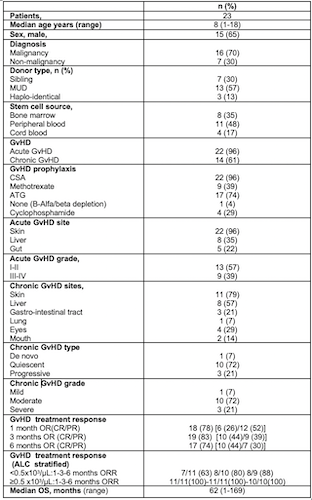
Patients, HSCT, GvHD and treatment characteristics are summarized in Table.
Twenty-two (96%) and 14 (61%) children were diagnosed with aGvHD and cGvHD, respectively. Acute GvHD involved skin, gut and liver in 22 (96%), 8 (35%) and 5 (22%) cases; 4 (17%), 4 (17%) and 1 (4%) patients had a simultaneous involvement of skin+liver+gut, skin+liver and skin+gut, respectively. Grade I-II and III-IV aGvHD was observed in 13 (57%) and 9 (39%) children, respectively.
Among patients with cGvHD, involvement of skin, gut , liver, lung, mouth and eyes was detected in 11 (79%), 3 (21%) , 8 (57%), 1(7%), 2 (14%) and 4 (29%) cases, respectively. Chronic GvHD was classified as mild, moderate and severe in 1 (7%), 10 (72%) and 3 (21%) patients.
ECP therapy was started in 10 (43%) patients for aGvHD and 13 (57%) for cGvHD.
Median ECP treatment duration was 3 months (1-29), with a median number of 16 ECP (2-74). ECP was associated with a new IS (MMF-Tacrolimus-Sirolimus), Jak2 inhibitor (Ruxolitinib), monoclonal antibodies (Rituximab or Basiliximab) and anti-TNFα in 11 (48%), 1 (4%), 5 (22%), in 5 (22%) patients, respectively. Overall response rates (ORR) evaluated at 1, 3 and 6 months were 78%, 83%, 74% and 67% ,88%,100% for aGvHD and cGvHD respectively.
Infectious complications consisted of viral reactivations bacterial, fungal infections in 4 (17%)(1 CMV, 2 ADV, 1 ADV+EBV), 10 (43%) and 1 (4%) patients, respectively. No relapse of underlying disease occurred and only 1 (4%) child with positive minimal measurable disease (MRD) responded to tyrosine kinase inhibitor.
Median overall survival (mOS) was not correlated with grade for aGvHD [grade I-II vs III-IV; not reached (NR) vs 24 months;HR:2.6;p=0.14) or cGvHD (mild+moderate vs severe; NR vs 24 months; HR.4.6;p=0.09) while for involvement of gut in aGvHD [18 months vs NR;HR:3.26;p=0.06] and liver in cGvHD [50 months vs NR;HR:61.3;p=0.056] there was a trend with shorter mOS.
There were 12 (52%) and 11 (48%) patients with absolute lymphocyte count (ALC)<0.5 and ≥0.5 x103/μL, respectively; ALC ≥0.5 x103/μL was significantly correlated with higher 1-month ORR [100%vs64%;p=0.04]and longer mOS (NR vs 24 months; HR:0.2; p=0.02) while 3 and 6 month-ORR were not influenced (100% vs 80%; p=0.31; 100% vs 89%; p=0.44).
Conclusion
ECP treatment is a safe and effective treatment for aGvHD and cGVHD in our pediatric population with superior ORR, decreased infection complications, and disease relapse compared to IS combination treatment for clinical practice as reported in the literature. Prospective trials are needed to validate our observation and further investigate the correlation of ALC with outcomes
Keyword(s): Children, Graft-versus-host disease (GVHD), Photopheresis
Abstract: PB1790
Type: Publication Only
Session title: Stem cell transplantation - Clinical
Background
Steroid refractory (SR) and dependent (SD) acute or chronic Graft versus Host Disease (aGvHD/cGvHD) are severe complications of allogenic Hematopoietic Stem Cell Transplantation (HSCT) with high morbidity and mortality rates.
Extracorporeal Photopheresis (ECP) is a therapeutic option with T-cell immunoregulatory activity but without ImmunoSuppressive (IS) effects.
Aims
We retrospectively evaluated efficacy and safety of ECP in our pediatric population.
Methods
We reviewed data from 23 children with SR or SD GvHD followed at our Institution since 2010.
ECP was performed by COBE SpectraTMand Therakos CELLEXTM systems; the schedule consisted of 2 consecutive days each week for 4-12 weeks with tapering to biweekly/monthly regimens, adjusted by patient response.
Complete and partial responses (CR and PR) were defined by resolution of all signs and symptoms of GvHD in every organ and by 1 grade improvement in 1 organ without any progression, respectively.
Results
Patients, HSCT, GvHD and treatment characteristics are summarized in Table.
Twenty-two (96%) and 14 (61%) children were diagnosed with aGvHD and cGvHD, respectively. Acute GvHD involved skin, gut and liver in 22 (96%), 8 (35%) and 5 (22%) cases; 4 (17%), 4 (17%) and 1 (4%) patients had a simultaneous involvement of skin+liver+gut, skin+liver and skin+gut, respectively. Grade I-II and III-IV aGvHD was observed in 13 (57%) and 9 (39%) children, respectively.
Among patients with cGvHD, involvement of skin, gut , liver, lung, mouth and eyes was detected in 11 (79%), 3 (21%) , 8 (57%), 1(7%), 2 (14%) and 4 (29%) cases, respectively. Chronic GvHD was classified as mild, moderate and severe in 1 (7%), 10 (72%) and 3 (21%) patients.
ECP therapy was started in 10 (43%) patients for aGvHD and 13 (57%) for cGvHD.
Median ECP treatment duration was 3 months (1-29), with a median number of 16 ECP (2-74). ECP was associated with a new IS (MMF-Tacrolimus-Sirolimus), Jak2 inhibitor (Ruxolitinib), monoclonal antibodies (Rituximab or Basiliximab) and anti-TNFα in 11 (48%), 1 (4%), 5 (22%), in 5 (22%) patients, respectively. Overall response rates (ORR) evaluated at 1, 3 and 6 months were 78%, 83%, 74% and 67% ,88%,100% for aGvHD and cGvHD respectively.
Infectious complications consisted of viral reactivations bacterial, fungal infections in 4 (17%)(1 CMV, 2 ADV, 1 ADV+EBV), 10 (43%) and 1 (4%) patients, respectively. No relapse of underlying disease occurred and only 1 (4%) child with positive minimal measurable disease (MRD) responded to tyrosine kinase inhibitor.
Median overall survival (mOS) was not correlated with grade for aGvHD [grade I-II vs III-IV; not reached (NR) vs 24 months;HR:2.6;p=0.14) or cGvHD (mild+moderate vs severe; NR vs 24 months; HR.4.6;p=0.09) while for involvement of gut in aGvHD [18 months vs NR;HR:3.26;p=0.06] and liver in cGvHD [50 months vs NR;HR:61.3;p=0.056] there was a trend with shorter mOS.
There were 12 (52%) and 11 (48%) patients with absolute lymphocyte count (ALC)<0.5 and ≥0.5 x103/μL, respectively; ALC ≥0.5 x103/μL was significantly correlated with higher 1-month ORR [100%vs64%;p=0.04]and longer mOS (NR vs 24 months; HR:0.2; p=0.02) while 3 and 6 month-ORR were not influenced (100% vs 80%; p=0.31; 100% vs 89%; p=0.44).

Conclusion
ECP treatment is a safe and effective treatment for aGvHD and cGVHD in our pediatric population with superior ORR, decreased infection complications, and disease relapse compared to IS combination treatment for clinical practice as reported in the literature. Prospective trials are needed to validate our observation and further investigate the correlation of ALC with outcomes
Keyword(s): Children, Graft-versus-host disease (GVHD), Photopheresis


