
Contributions
Abstract: PB1764
Type: Publication Only
Session title: Sickle cell disease
Background
The hallmark of sickle cell disease (SCD) is hemoglobin S (HbS) polymerization upon deoxygenation, resulting in red blood cell (RBC) sickling, hemolysis, and vaso-occlusion. Exacerbating the pathogenesis of SCD, the HbS RBC has increased (↑) levels of 2,3-diphosphoglycerate (2,3-DPG), resulting in reduced (↓) Hb oxygen affinity (↑P50), and ↓ATP, essential for RBC homeostasis. FT-4202 is a selective, and orally bioavailable allosteric activator of erythrocyte pyruvate kinase (PKR) that increases PKR activity, resulting in ↓2,3-DPG levels and ↑ATP levels in RBCs. Preliminary data from an ongoing phase 1 study (NCT03815695) indicate that FT 4202 is well-tolerated, has no effect on steroidogenesis, and exhibits linear and time-independent pharmacokinetics and associated pharmacodynamic responses (↓2,3-DPG and ↑ATP). Treatment of patients with SCD for 14 days with once-daily FT-4202 resulted in ↑Hb O2 affinity, ↓RBC sickling, improved RBC deformability, and improved hematologic and hemolytic parameters (Brown, ASH2020_ abstract_#134269).
Aims
Hibiscus (NCT04624659) is a phase 2/3, randomized, double-blind, placebo-controlled global study to investigate the safety and efficacy of FT-4202 in patients with SCD.
Methods
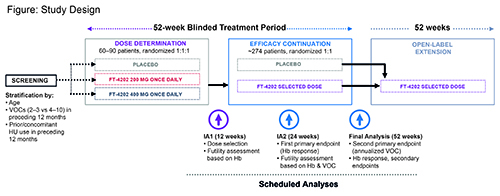
Adult and adolescent patients with SCD (all genotypes) will be enrolled in a Dose Determination (DD) group and an Efficacy Continuation (EC) group using an adaptive design. Eligible patients must have had ≥2 vaso-occlusive crises (VOCs) in the past year, baseline Hb ≥5.5 and ≤10 g/dL, and if receiving hydroxyurea (HU), be on stable therapy for the previous 90 days. Patients with >10 VOCs in the past year, hospitalized for sickle cell crisis/other vaso-occlusive event within 14 days of consent, receiving routine RBC transfusions, significant hepatic/renal dysfunction, history of unstable or deteriorating cardiac or pulmonary disease, or overt stroke within 2 years will be excluded. Co-primary endpoints are Hb response rate (RR) at Week 24 (increase from baseline >1 g/dL) measured as the percentage of treated population achieving the endpoint, and annualized VOC rate during the blinded treatment period based on adjudicated VOC review. Secondary endpoints include measures of hemolysis, time to first VOC, and the Patient Reported Outcome Measurement Information System fatigue scale. Safety endpoints will also be assessed. Patients will be stratified by number of VOCs in the preceding 12 months, prior/concomitant HU use and age, and randomized 1:1:1 to 200 or 400 mg (once-daily) FT-4202, or placebo in the DD group. At interim analysis (IA) 1, one FT-4202 dose level will be selected based on safety and Hb RR at Week 12 in the first 60 patients. EC group patients will be randomized 1:1 to the selected FT-4202 dose or placebo. Once 110 patients randomized to the selected dose or placebo complete 24-weeks blinded treatment or drop out, IA2 will assess Hb RR (first primary endpoint). At final analysis (52-weeks blinded treatment) annualized VOC (second primary endpoint), 24-week Hb RR and all secondary endpoints will be evaluated. Patients may then enter a 52-week open-label extension period at the selected FT-4202 dose. Futility assessments will be conducted (Weeks 12 and 24).
Results
Recruitment is ongoing; planned enrolment: ~344 patients with SCD (DD group, n=60–90; EC group, n~274).
Conclusion
This study will evaluate the safety and efficacy of oral, once-daily FT-4202 in adult and adolescent patients with SCD.
Keyword(s): Sickle cell disease
Abstract: PB1764
Type: Publication Only
Session title: Sickle cell disease
Background
The hallmark of sickle cell disease (SCD) is hemoglobin S (HbS) polymerization upon deoxygenation, resulting in red blood cell (RBC) sickling, hemolysis, and vaso-occlusion. Exacerbating the pathogenesis of SCD, the HbS RBC has increased (↑) levels of 2,3-diphosphoglycerate (2,3-DPG), resulting in reduced (↓) Hb oxygen affinity (↑P50), and ↓ATP, essential for RBC homeostasis. FT-4202 is a selective, and orally bioavailable allosteric activator of erythrocyte pyruvate kinase (PKR) that increases PKR activity, resulting in ↓2,3-DPG levels and ↑ATP levels in RBCs. Preliminary data from an ongoing phase 1 study (NCT03815695) indicate that FT 4202 is well-tolerated, has no effect on steroidogenesis, and exhibits linear and time-independent pharmacokinetics and associated pharmacodynamic responses (↓2,3-DPG and ↑ATP). Treatment of patients with SCD for 14 days with once-daily FT-4202 resulted in ↑Hb O2 affinity, ↓RBC sickling, improved RBC deformability, and improved hematologic and hemolytic parameters (Brown, ASH2020_ abstract_#134269).
Aims
Hibiscus (NCT04624659) is a phase 2/3, randomized, double-blind, placebo-controlled global study to investigate the safety and efficacy of FT-4202 in patients with SCD.
Methods
Adult and adolescent patients with SCD (all genotypes) will be enrolled in a Dose Determination (DD) group and an Efficacy Continuation (EC) group using an adaptive design. Eligible patients must have had ≥2 vaso-occlusive crises (VOCs) in the past year, baseline Hb ≥5.5 and ≤10 g/dL, and if receiving hydroxyurea (HU), be on stable therapy for the previous 90 days. Patients with >10 VOCs in the past year, hospitalized for sickle cell crisis/other vaso-occlusive event within 14 days of consent, receiving routine RBC transfusions, significant hepatic/renal dysfunction, history of unstable or deteriorating cardiac or pulmonary disease, or overt stroke within 2 years will be excluded. Co-primary endpoints are Hb response rate (RR) at Week 24 (increase from baseline >1 g/dL) measured as the percentage of treated population achieving the endpoint, and annualized VOC rate during the blinded treatment period based on adjudicated VOC review. Secondary endpoints include measures of hemolysis, time to first VOC, and the Patient Reported Outcome Measurement Information System fatigue scale. Safety endpoints will also be assessed. Patients will be stratified by number of VOCs in the preceding 12 months, prior/concomitant HU use and age, and randomized 1:1:1 to 200 or 400 mg (once-daily) FT-4202, or placebo in the DD group. At interim analysis (IA) 1, one FT-4202 dose level will be selected based on safety and Hb RR at Week 12 in the first 60 patients. EC group patients will be randomized 1:1 to the selected FT-4202 dose or placebo. Once 110 patients randomized to the selected dose or placebo complete 24-weeks blinded treatment or drop out, IA2 will assess Hb RR (first primary endpoint). At final analysis (52-weeks blinded treatment) annualized VOC (second primary endpoint), 24-week Hb RR and all secondary endpoints will be evaluated. Patients may then enter a 52-week open-label extension period at the selected FT-4202 dose. Futility assessments will be conducted (Weeks 12 and 24).
Results
Recruitment is ongoing; planned enrolment: ~344 patients with SCD (DD group, n=60–90; EC group, n~274).

Conclusion
This study will evaluate the safety and efficacy of oral, once-daily FT-4202 in adult and adolescent patients with SCD.
Keyword(s): Sickle cell disease


