
Contributions
Abstract: PB1761
Type: Publication Only
Session title: Sickle cell disease
Background
In Sickle Cell Disease (SCD) sickle hemoglobin (HbS) can polymerize in its deoxygenated form, leading to perturbations of Red Blood Cells (RBC) biochemical, morphological, and mechanical properties. RBC membrane damage due to hypoxia-induced polymerization causes hemolysis leading to hemolytic anemia and other complications linked to elevated cell-free Hb levels and heme toxicity.
Aims
The FDA recently approved voxelotor (Oxbryta; Global Blood Therapeutics) for treatment of SCD in patients 12 years of age and older, shown to increase patient Hb levels in a pivotal phase 3 trial. In this study, we assessed the effect of GBT1118, a voxelotor (Oxbryta) analog on hypoxia-induced RBC membrane damage (lethal and sublethal) using standardized assays.
Methods
Blood was from steady state homozygous SS SCD patients of Children’s Hospital of Michigan (n=4; HbS=62-85%). Informed consent was obtained per protocol approved by Wayne State University IRB. RBC Mechanical Fragility (MF), a metric indicative of existing RBC membrane damage and prospective hemolysis, was measured using a proprietary system comprising an electromagnetic bead mill and fiber-optic spectrophotometry detection. Non-invasive probing of sample agitated with a magnetic bead at 5 Hz allowed determination of MF indices (MFI), representative of RBC resistance to induced hemolysis. MFIs were assessed for most labile RBC fraction (induced hemolysis ab. 20%, MFI_1) and for total induced hemolysis over 10 minutes agitation time (MFI_10). RBCs were assessed at normoxia, before and after three cycles of hypoxia, with deoxygenation induced via protocatechuate/protocatechuate dioxygenase oxygen scavenging system (< 5% oxygenated-Hb) and re-oxygenation under normoxic conditions (close to 100% RBC recovery).
Results
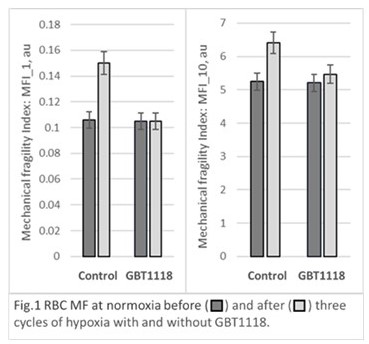
Whole blood samples supplemented with 1 mM GBT1118 in DMSO (0.9% final concentration) were compared in-vitro to those with equal volume of DMSO-containing buffer. For control samples, three cycles of hypoxia with follow-up reoxygenation resulted in significant increase in RBC MF indices (MFI) for all samples with changes for labile fraction being more pronounced. Supplementation with GBT1118 did not result in statistically significant changes in RBC MFI_1 or MFI_10 as compared to control samples pre-hypoxia. Following GBT1118 treatment, MFI_1 and MFI_10 showed significantly less increase after the hypoxia-reoxygenation cycles with the averages unchanged from MFIs’ pre-hypoxia values. (Figure 1). Average (mean ±SD), for the 4 samples, percent change from pre-hypoxia after the 3 cycles of hypoxia was 47%±28% (MFI_1) and 25%±20% (MFI_10) for the control samples compared to 0%±20% (MF_1) and 5%±7% for (MFI_10) for samples supplemented with GBT1118. Changes in pre-existing (existing prior to stress application) sample hemolysis due to hypoxia cycles were not significant for any of the samples.
Conclusion
Cycles of severe hypoxia followed significantly damage RBC membranes making cells more susceptible to lysis under flow-induced mechanical stress. With no history of hypoxia, GBT1118 did not affect membrane stability under normoxic conditions. However, when present during hypoxia, GBT1118 significantly reduced membrane damage as shown by reduced RBC propensity to lyse. That highlights the potential of GBT1118, a voxelotor (Oxbryta) analog, to prevent sub-lethal RBC membrane damage likely leading in increased RBC survival in circulation
Keyword(s): Red blood cell, Sickle cell disease, Therapy
Abstract: PB1761
Type: Publication Only
Session title: Sickle cell disease
Background
In Sickle Cell Disease (SCD) sickle hemoglobin (HbS) can polymerize in its deoxygenated form, leading to perturbations of Red Blood Cells (RBC) biochemical, morphological, and mechanical properties. RBC membrane damage due to hypoxia-induced polymerization causes hemolysis leading to hemolytic anemia and other complications linked to elevated cell-free Hb levels and heme toxicity.
Aims
The FDA recently approved voxelotor (Oxbryta; Global Blood Therapeutics) for treatment of SCD in patients 12 years of age and older, shown to increase patient Hb levels in a pivotal phase 3 trial. In this study, we assessed the effect of GBT1118, a voxelotor (Oxbryta) analog on hypoxia-induced RBC membrane damage (lethal and sublethal) using standardized assays.
Methods
Blood was from steady state homozygous SS SCD patients of Children’s Hospital of Michigan (n=4; HbS=62-85%). Informed consent was obtained per protocol approved by Wayne State University IRB. RBC Mechanical Fragility (MF), a metric indicative of existing RBC membrane damage and prospective hemolysis, was measured using a proprietary system comprising an electromagnetic bead mill and fiber-optic spectrophotometry detection. Non-invasive probing of sample agitated with a magnetic bead at 5 Hz allowed determination of MF indices (MFI), representative of RBC resistance to induced hemolysis. MFIs were assessed for most labile RBC fraction (induced hemolysis ab. 20%, MFI_1) and for total induced hemolysis over 10 minutes agitation time (MFI_10). RBCs were assessed at normoxia, before and after three cycles of hypoxia, with deoxygenation induced via protocatechuate/protocatechuate dioxygenase oxygen scavenging system (< 5% oxygenated-Hb) and re-oxygenation under normoxic conditions (close to 100% RBC recovery).
Results
Whole blood samples supplemented with 1 mM GBT1118 in DMSO (0.9% final concentration) were compared in-vitro to those with equal volume of DMSO-containing buffer. For control samples, three cycles of hypoxia with follow-up reoxygenation resulted in significant increase in RBC MF indices (MFI) for all samples with changes for labile fraction being more pronounced. Supplementation with GBT1118 did not result in statistically significant changes in RBC MFI_1 or MFI_10 as compared to control samples pre-hypoxia. Following GBT1118 treatment, MFI_1 and MFI_10 showed significantly less increase after the hypoxia-reoxygenation cycles with the averages unchanged from MFIs’ pre-hypoxia values. (Figure 1). Average (mean ±SD), for the 4 samples, percent change from pre-hypoxia after the 3 cycles of hypoxia was 47%±28% (MFI_1) and 25%±20% (MFI_10) for the control samples compared to 0%±20% (MF_1) and 5%±7% for (MFI_10) for samples supplemented with GBT1118. Changes in pre-existing (existing prior to stress application) sample hemolysis due to hypoxia cycles were not significant for any of the samples.

Conclusion
Cycles of severe hypoxia followed significantly damage RBC membranes making cells more susceptible to lysis under flow-induced mechanical stress. With no history of hypoxia, GBT1118 did not affect membrane stability under normoxic conditions. However, when present during hypoxia, GBT1118 significantly reduced membrane damage as shown by reduced RBC propensity to lyse. That highlights the potential of GBT1118, a voxelotor (Oxbryta) analog, to prevent sub-lethal RBC membrane damage likely leading in increased RBC survival in circulation
Keyword(s): Red blood cell, Sickle cell disease, Therapy


