
Contributions
Abstract: PB1750
Type: Publication Only
Session title: Quality of life, palliative care, ethics and health economics
Background
Myelofibrosis (MF) is a rare (annual incidence estimated to be 1/100,000 in Europe), chronic hematologic disorder associated with morbidity and mortality as well as the risk of evolution to acute myeloid leukemia. Ruxolitinib (Jakavi®, Novartis) is the first JAK 1/2 inhibitor approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) in 2011 for the treatment of patients with MF. Ruxolitinib is considered a high-cost and life-time treatment. UK-based estimates of the cost of treatment are in the region of £43,000 per patient, per year. The work was completed in partial fulfilment of an MSc in Applied Economics for Healthcare Decision Making from the University of South Wales. The research was deemed as low risk and as such was reviewed by the Low Risk Ethical procedure at the Faculty of Life Science and Education, University of South Wales and granted approval.
Aims
Against the background of the challenge of treatments for rare diseases reaching cost-effectiveness thresholds, this study identified, synthesized and appraised the available evidence on the cost-effectiveness of ruxolitinib in the treatment of MF.
Methods
A systematic approach was taken to conducting the literature review. Databases searched included PubMed, EMBASE, MEDLINE, and the Cochrane Library based on search terms informed by Population, Intervention, Comparator and Outcomes (PICO): myelofibrosis, ruxolitinib, best available therapy/standard of care, and cost-effectiveness/cost-utility/pharmacoeconomics. The search was limited to studies published in the English language. Narrative synthesis was used to evaluate included studies and the CHEERS checklist to explore quality of reporting of the cost-effectiveness analysis.
Results
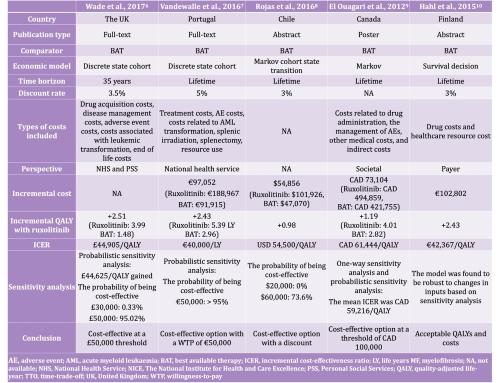
The narrative synthesis included five studies conducted in the UK, Portugal, Chile, Canada, and Finland. All cost-effectiveness analyses used data obtained from the same two large, randomized controlled, double-blind, phase III studies (COMFORT-I and -II) that led to the regulatory approval of ruxolitinib. Ruxolitinib was compared to best available therapy (BAT), including hydroxyurea, no medication, and prednisone/prednisolone. Perspectives and included costs varied among analyses. Markov models and discrete state cohort models were used to evaluate cost-effectiveness and clinical benefit was measured in quality-adjusted life years (QALY) or life years (LY) gained.
These analyses estimated the base case incremental cost-effectiveness ratios (ICER) per QALY of (converted into USD, if appropriate, at the historic average annual exchange rate) 44,905 GBP in the UK (in 2013; USD 70,2262), 40,000 EUR in Portugal (in 2016; USD 44,272), 54,500 USD in Chile (in 2016), 61,444 CAD in Canada (in 2012; USD 61,474), and 42,367 EUR in Finland (in 2015; USD 42,027). Based upon the cost-effectiveness thresholds applied in each of these countries, ruxolitinib was found to be universally cost-effective, albeit with price adjustments as part of the wider pricing and reimbursement processes used in these countries.
Conclusion
Ruxolitinib was found to be cost-effective for the treatment of patients with myelofibrosis informed by different types of models and from different perspectives; however, there was some uncertainty around available data due to a very limited data source.
Keyword(s):
Abstract: PB1750
Type: Publication Only
Session title: Quality of life, palliative care, ethics and health economics
Background
Myelofibrosis (MF) is a rare (annual incidence estimated to be 1/100,000 in Europe), chronic hematologic disorder associated with morbidity and mortality as well as the risk of evolution to acute myeloid leukemia. Ruxolitinib (Jakavi®, Novartis) is the first JAK 1/2 inhibitor approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) in 2011 for the treatment of patients with MF. Ruxolitinib is considered a high-cost and life-time treatment. UK-based estimates of the cost of treatment are in the region of £43,000 per patient, per year. The work was completed in partial fulfilment of an MSc in Applied Economics for Healthcare Decision Making from the University of South Wales. The research was deemed as low risk and as such was reviewed by the Low Risk Ethical procedure at the Faculty of Life Science and Education, University of South Wales and granted approval.
Aims
Against the background of the challenge of treatments for rare diseases reaching cost-effectiveness thresholds, this study identified, synthesized and appraised the available evidence on the cost-effectiveness of ruxolitinib in the treatment of MF.
Methods
A systematic approach was taken to conducting the literature review. Databases searched included PubMed, EMBASE, MEDLINE, and the Cochrane Library based on search terms informed by Population, Intervention, Comparator and Outcomes (PICO): myelofibrosis, ruxolitinib, best available therapy/standard of care, and cost-effectiveness/cost-utility/pharmacoeconomics. The search was limited to studies published in the English language. Narrative synthesis was used to evaluate included studies and the CHEERS checklist to explore quality of reporting of the cost-effectiveness analysis.
Results
The narrative synthesis included five studies conducted in the UK, Portugal, Chile, Canada, and Finland. All cost-effectiveness analyses used data obtained from the same two large, randomized controlled, double-blind, phase III studies (COMFORT-I and -II) that led to the regulatory approval of ruxolitinib. Ruxolitinib was compared to best available therapy (BAT), including hydroxyurea, no medication, and prednisone/prednisolone. Perspectives and included costs varied among analyses. Markov models and discrete state cohort models were used to evaluate cost-effectiveness and clinical benefit was measured in quality-adjusted life years (QALY) or life years (LY) gained.
These analyses estimated the base case incremental cost-effectiveness ratios (ICER) per QALY of (converted into USD, if appropriate, at the historic average annual exchange rate) 44,905 GBP in the UK (in 2013; USD 70,2262), 40,000 EUR in Portugal (in 2016; USD 44,272), 54,500 USD in Chile (in 2016), 61,444 CAD in Canada (in 2012; USD 61,474), and 42,367 EUR in Finland (in 2015; USD 42,027). Based upon the cost-effectiveness thresholds applied in each of these countries, ruxolitinib was found to be universally cost-effective, albeit with price adjustments as part of the wider pricing and reimbursement processes used in these countries.

Conclusion
Ruxolitinib was found to be cost-effective for the treatment of patients with myelofibrosis informed by different types of models and from different perspectives; however, there was some uncertainty around available data due to a very limited data source.
Keyword(s):


