
Contributions
Abstract: PB1723
Type: Publication Only
Session title: Myeloproliferative neoplasms - Clinical
Background
Myelofibrosis (MF) is a myeloproliferative neoplasm (MPN) that can develop de novo (primary MF) or from the progression of antecedent MPNs, in particular polycythemia vera (PPV-MF) or essential thrombocythemia (PET-MF). Ruxolitinib (RUX), a first-in-class JAK1/JAK2 inhibitor, is the standard of care for MF and demonstrated superiority over placebo (COMFORT-I) and best available therapy (COMFORT-II). However, RUX is not curative, cytopenias remain a challenge and some patients (pts) lose response or discontinue treatment due to adverse events. RUX combined with novel agents may offer superior disease control and transformative clinical benefits.
Aims
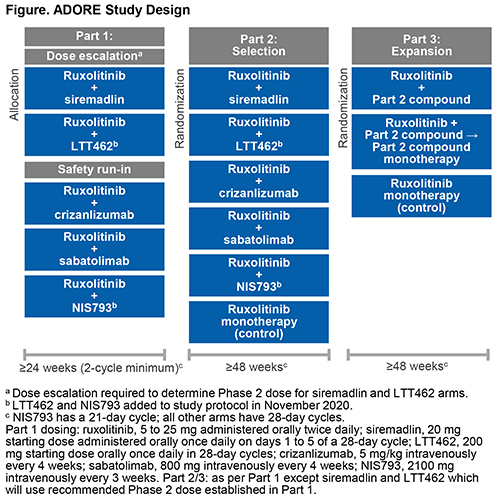
ADORE (NCT04097821) is a 3-part, open-label, multicenter, phase 1/2, platform study that will assess the safety and efficacy of RUX in combination with 5 novel compounds for the treatment of MF: siremadlin (HDM2 inhibitor), crizanlizumab (P-selectin inhibitor), sabatolimab (TIM-3 inhibitor) and, recently added to the study, LTT462 (ERK1/2 inhibitor) and NIS793 (anti-TGFβ antibody).
Methods
The study includes pts ≥18 years with primary MF, PPV-MF, or PET-MF, splenomegaly (a palpable spleen ≥5 cm from the left costal margin or spleen volume ≥450 cm3 by MRI or CT scan), hemoglobin level <11 g/dL, and platelet count ≥75 × 109/L (≥50 × 109/L in Parts 2/3). Pts must have received RUX for ≥24 weeks and at a stable dose for ≥8 weeks prior to study entry.
Part 1 (phase 1b; Safety) includes a dose escalation arm to determine the recommended phase 2 dose for siremadlin + RUX and LTT462 + RUX, and a safety run-in for RUX + crizanlizumab, RUX + sabatolimab and RUX + NIS793. RUX will be administered at the same stable dose used prior to study entry. Pts will be treated for ≥24 weeks (8 cycles in NIS793 arm or 6 cycles in other arms). Part 1 primary endpoint is the incidence of dose-limiting toxicities within the first 2 cycles. Combinations evaluated as safe and tolerable in Part 1 may be selected for Part 2.
In Part 2 (phase 2; Selection), pts will be randomized to one of the selected combinations or RUX monotherapy (control) and treated for ≥48 weeks. After all Part 2 pts have completed 24 weeks of treatment, a futility interim analysis will determine which treatment will proceed to Part 3.
In Part 3 (phase 2; Expansion), pts will be randomized to the combination treatment, RUX cessation, or RUX monotherapy and treated for ≥48 weeks. Pts in the RUX cessation arm will be treated with combination therapy for 12 weeks, followed by novel agent monotherapy.
The primary endpoint for Parts 2 and 3 is the response rate (a composite of anemia improvement [increase in hemoglobin of ≥1.5 g/dL], no spleen volume progression, and no symptom worsening) at the end of cycle 8 (NIS793 arms) or cycle 6 (other arms) (24 weeks). The study will end 24 months after the last pt has initiated Part 3. A total of 240 patients are planned to be enrolled.
Results
Trial in progress.
Conclusion
As of January 2021, the study is open for enrolling into Part 1.
Keyword(s): Clinical trial, Janus Kinase inhibitor, Myelofibrosis, Ruxolitinib
Abstract: PB1723
Type: Publication Only
Session title: Myeloproliferative neoplasms - Clinical
Background
Myelofibrosis (MF) is a myeloproliferative neoplasm (MPN) that can develop de novo (primary MF) or from the progression of antecedent MPNs, in particular polycythemia vera (PPV-MF) or essential thrombocythemia (PET-MF). Ruxolitinib (RUX), a first-in-class JAK1/JAK2 inhibitor, is the standard of care for MF and demonstrated superiority over placebo (COMFORT-I) and best available therapy (COMFORT-II). However, RUX is not curative, cytopenias remain a challenge and some patients (pts) lose response or discontinue treatment due to adverse events. RUX combined with novel agents may offer superior disease control and transformative clinical benefits.
Aims
ADORE (NCT04097821) is a 3-part, open-label, multicenter, phase 1/2, platform study that will assess the safety and efficacy of RUX in combination with 5 novel compounds for the treatment of MF: siremadlin (HDM2 inhibitor), crizanlizumab (P-selectin inhibitor), sabatolimab (TIM-3 inhibitor) and, recently added to the study, LTT462 (ERK1/2 inhibitor) and NIS793 (anti-TGFβ antibody).
Methods
The study includes pts ≥18 years with primary MF, PPV-MF, or PET-MF, splenomegaly (a palpable spleen ≥5 cm from the left costal margin or spleen volume ≥450 cm3 by MRI or CT scan), hemoglobin level <11 g/dL, and platelet count ≥75 × 109/L (≥50 × 109/L in Parts 2/3). Pts must have received RUX for ≥24 weeks and at a stable dose for ≥8 weeks prior to study entry.
Part 1 (phase 1b; Safety) includes a dose escalation arm to determine the recommended phase 2 dose for siremadlin + RUX and LTT462 + RUX, and a safety run-in for RUX + crizanlizumab, RUX + sabatolimab and RUX + NIS793. RUX will be administered at the same stable dose used prior to study entry. Pts will be treated for ≥24 weeks (8 cycles in NIS793 arm or 6 cycles in other arms). Part 1 primary endpoint is the incidence of dose-limiting toxicities within the first 2 cycles. Combinations evaluated as safe and tolerable in Part 1 may be selected for Part 2.
In Part 2 (phase 2; Selection), pts will be randomized to one of the selected combinations or RUX monotherapy (control) and treated for ≥48 weeks. After all Part 2 pts have completed 24 weeks of treatment, a futility interim analysis will determine which treatment will proceed to Part 3.
In Part 3 (phase 2; Expansion), pts will be randomized to the combination treatment, RUX cessation, or RUX monotherapy and treated for ≥48 weeks. Pts in the RUX cessation arm will be treated with combination therapy for 12 weeks, followed by novel agent monotherapy.
The primary endpoint for Parts 2 and 3 is the response rate (a composite of anemia improvement [increase in hemoglobin of ≥1.5 g/dL], no spleen volume progression, and no symptom worsening) at the end of cycle 8 (NIS793 arms) or cycle 6 (other arms) (24 weeks). The study will end 24 months after the last pt has initiated Part 3. A total of 240 patients are planned to be enrolled.
Results
Trial in progress.

Conclusion
As of January 2021, the study is open for enrolling into Part 1.
Keyword(s): Clinical trial, Janus Kinase inhibitor, Myelofibrosis, Ruxolitinib


