
Contributions
Abstract: PB1720
Type: Publication Only
Session title: Myeloproliferative neoplasms - Clinical
Background
Many neoplasms including myelofibrosis (MF) are associated with activation of transcription factors that regulate oncogenic processes. These may respond to inhibition of bromodomain and extra-terminal domain (BET) protein. In the first-in-human study INCB 57643-101, the BET inhibitor INCB057643 was generally safe and tolerable as monotherapy, and demonstrated preliminary efficacy in a small number of patients with MF, as monotherapy or combined with the JAK inhibitor, ruxolitinib (Falchook G, et al. Clin Cancer Res. 2020).
Aims
This phase 1, open-label, two-part dose confirmation and expansion study further evaluates the safety and tolerability of INCB057643 monotherapy in patients with relapsed/refractory MF (INCB 57643-103; NCT04279847).
Methods
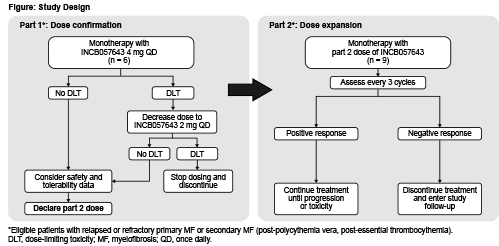
Eligible patients must be aged ≥18 years with histologically confirmed primary or secondary (post-polycythemia vera, post-essential thrombocythemia) MF, have received ≥1 prior therapy including ruxolitinib, have no known clinically beneficial therapy available, have intermediate-2/high risk disease by Dynamic International Prognostic Scoring System, have ECOG PS 0–2, life expectancy ≥24 weeks, and willing to provide a bone marrow biopsy and/or aspirate at baseline (or archival sample obtained after most recent therapy). Exclusion criteria include prior BET inhibitor or anticancer treatment within specified intervals before first dose; concurrent anticancer therapy; allogeneic hematopoietic stem cell transplant (allo-HSCT) ≤6 months before enrollment; active graft versus host disease or immunosuppressive therapy after allo-HSCT ≤2 weeks before first-dose; significant and uncontrolled disease (eg, gastrointestinal, cardiovascular); history of bleeding disorders, high risk of bleeding, or abnormal hematologic, hepatic, renal, coagulation, or metabolic laboratory values. In part 1, ≤6 patients will receive oral INCB057643 4 mg once-daily continuously (Figure). Doses will be deemed tolerable if ≤2 patients experience dose-limiting toxicities (DLTs) and ≤2 patients discontinue due to treatment-related adverse events (TRAEs) during the DLT evaluation period. Part 2 will further characterize safety and tolerability as well as evaluate preliminary efficacy of INCB057643 in ≤9 patients. The starting dose will be 4 mg once-daily if tolerated in part 1, and 2 mg once-daily if not. Treatment may continue if clinically beneficial and discontinuation criteria are not met. The primary objective is to determine the safety and tolerability of INCB057643 monotherapy. Secondary objectives are to evaluate anemia response by International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet Consensus Report (ELN), transfusion dependence, spleen volume, rate and duration of spleen response by IWG-MRT and ELN, and impact on quality of life. Patients will be assessed every 3 cycles and will receive follow-up for safety for 30–35 days after last dose. Sites are opening in the United States and Europe.
Results
Not applicable
Conclusion
Not applicable
Keyword(s): Epigenetic, Myelofibrosis, Myeloproliferative disorder
Abstract: PB1720
Type: Publication Only
Session title: Myeloproliferative neoplasms - Clinical
Background
Many neoplasms including myelofibrosis (MF) are associated with activation of transcription factors that regulate oncogenic processes. These may respond to inhibition of bromodomain and extra-terminal domain (BET) protein. In the first-in-human study INCB 57643-101, the BET inhibitor INCB057643 was generally safe and tolerable as monotherapy, and demonstrated preliminary efficacy in a small number of patients with MF, as monotherapy or combined with the JAK inhibitor, ruxolitinib (Falchook G, et al. Clin Cancer Res. 2020).
Aims
This phase 1, open-label, two-part dose confirmation and expansion study further evaluates the safety and tolerability of INCB057643 monotherapy in patients with relapsed/refractory MF (INCB 57643-103; NCT04279847).
Methods
Eligible patients must be aged ≥18 years with histologically confirmed primary or secondary (post-polycythemia vera, post-essential thrombocythemia) MF, have received ≥1 prior therapy including ruxolitinib, have no known clinically beneficial therapy available, have intermediate-2/high risk disease by Dynamic International Prognostic Scoring System, have ECOG PS 0–2, life expectancy ≥24 weeks, and willing to provide a bone marrow biopsy and/or aspirate at baseline (or archival sample obtained after most recent therapy). Exclusion criteria include prior BET inhibitor or anticancer treatment within specified intervals before first dose; concurrent anticancer therapy; allogeneic hematopoietic stem cell transplant (allo-HSCT) ≤6 months before enrollment; active graft versus host disease or immunosuppressive therapy after allo-HSCT ≤2 weeks before first-dose; significant and uncontrolled disease (eg, gastrointestinal, cardiovascular); history of bleeding disorders, high risk of bleeding, or abnormal hematologic, hepatic, renal, coagulation, or metabolic laboratory values. In part 1, ≤6 patients will receive oral INCB057643 4 mg once-daily continuously (Figure). Doses will be deemed tolerable if ≤2 patients experience dose-limiting toxicities (DLTs) and ≤2 patients discontinue due to treatment-related adverse events (TRAEs) during the DLT evaluation period. Part 2 will further characterize safety and tolerability as well as evaluate preliminary efficacy of INCB057643 in ≤9 patients. The starting dose will be 4 mg once-daily if tolerated in part 1, and 2 mg once-daily if not. Treatment may continue if clinically beneficial and discontinuation criteria are not met. The primary objective is to determine the safety and tolerability of INCB057643 monotherapy. Secondary objectives are to evaluate anemia response by International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet Consensus Report (ELN), transfusion dependence, spleen volume, rate and duration of spleen response by IWG-MRT and ELN, and impact on quality of life. Patients will be assessed every 3 cycles and will receive follow-up for safety for 30–35 days after last dose. Sites are opening in the United States and Europe.
Results
Not applicable

Conclusion
Not applicable
Keyword(s): Epigenetic, Myelofibrosis, Myeloproliferative disorder


