
Contributions
Abstract: PB1719
Type: Publication Only
Session title: Myeloproliferative neoplasms - Clinical
Background
Polycythaemia Vera (PV), a myeloproliferative neoplasm (MPN), is usually diagnosed in patients ≥60 years. However, 15-20% of PV patients are less than 40 years of age at diagnosis. MPN patients have increased risk for pregnancy-related complications (miscarriage, thrombosis, haemorrhage, intrauterine growth restriction, pre-eclampsia and stillbirths). Hence the importance of a correct management during this time, including the employment of aspirin, low molecular weight heparin (LMWH), venesection and cytoreductive therapy when needed. When cytoreduction is necessary, in PV patients wishing to conceive, interferon alfa is the choice. The development of pegylated formulations, including ropeginterferon alfa-2b (Besremi®), has resulted in improved tolerability and easier administration.
Aims
To describe the clinical and analitical evolution of a PV patient treated with novel ropeginterferon alfa-2b during pregnancy.
Methods
We present the case of a 32-year-old woman diagnosed of PV in December 2018 and followed up at Guy’s Hospital, who was on ropeginterferon alpha-2b during pregnancy, with adequate control and no complications.
Results
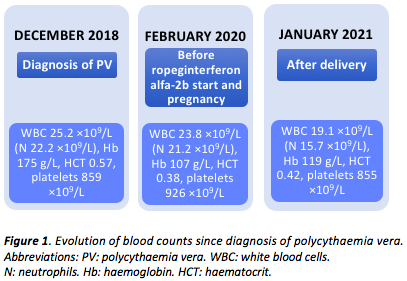
In December 2018, at the age of 29, she presented with numbness and intermittent paraesthesia in the right side of her face and body. She had had previous history of skin rash and bilateral conjunctival congestion. She had no history of thrombotic events. Neurological causes of her hyperviscosity syndrome were ruled out. Her leukocyte count was 25.2 ×109/L (neutrophils 22.2 ×109/L), with haemoglobin (Hb) of 175 g/L, haematocrit (Hct) of 0.57 and platelets of 859 ×109/L. Abdominal ultrasound scan showed splenomegaly (16 cm). JAK2 V617F mutation was detected and PV, with bone marrow (BM) features of panmyelosis, no immature precursors and grade 1 fibrosis, was diagnosed according to WHO criteria.
She had several venesections and started aspirin 150 mg/day. Due to myeloproliferative features, treatment with peginterferon alfa-2a (Pegasys®), 90 mcg weekly, was added, with later increases up to 225 mcg weekly.
As she had no major response to peginterferon alfa-2a, with persistent high leukocyte and platelet counts (leukocytes 23.8 ×109/L, neutrophils 21.2 ×109/L, Hb 107 g/L, Hct 0.38 and platelets 926 ×109/L), teardrop cells in the blood film and enlarged spleen (23 cm), in February 2020 another BM was performed, excluding progression to myelofibrosis. During this time she had the desire to concieve, but could not become pregnant. She was then switched to ropeginterferon alfa-2b, 350 mcg every other week.
In March 2020 she conceived, continuing ropeginterferon alfa-2b, as well as thromboprophylaxis with aspirin and enoxaparin 40 mg/day. She had several venesections during pregnancy, in order to maintain Hct <0.38 per BSH guidelines. She underwent local follow up with no special symptoms or signs of abnormal fetal development. Ultrasound scan in the third trimester demonstrated stability in spleen size.
The baby was delivered on 14th December 2020, at 38 weeks of gestation (weight 2.8 kg). The patient’s most recent blood control in January 2021, after delivery, showed a leukocyte count of 19.1 ×109/L (neutrophils 15.7 ×109/L), Hb of 119 g/L, Hct of 0.42 and platelet count of 855 ×109/L.
Conclusion
To our knowledge, this is the first case in which ropeginterferon alfa-2b is used in a pregnant PV patient and also the first reported case where a patient with inadequate disease control with peginterferon alfa-2a attained sufficient response with ropeginterferon alfa-2b.
An informed consent has been obtained from the patient to write this abstract.
Keyword(s): Myeloproliferative disorder, Pegylated Interferon, Polycythemia vera, Pregnancy
Abstract: PB1719
Type: Publication Only
Session title: Myeloproliferative neoplasms - Clinical
Background
Polycythaemia Vera (PV), a myeloproliferative neoplasm (MPN), is usually diagnosed in patients ≥60 years. However, 15-20% of PV patients are less than 40 years of age at diagnosis. MPN patients have increased risk for pregnancy-related complications (miscarriage, thrombosis, haemorrhage, intrauterine growth restriction, pre-eclampsia and stillbirths). Hence the importance of a correct management during this time, including the employment of aspirin, low molecular weight heparin (LMWH), venesection and cytoreductive therapy when needed. When cytoreduction is necessary, in PV patients wishing to conceive, interferon alfa is the choice. The development of pegylated formulations, including ropeginterferon alfa-2b (Besremi®), has resulted in improved tolerability and easier administration.
Aims
To describe the clinical and analitical evolution of a PV patient treated with novel ropeginterferon alfa-2b during pregnancy.
Methods
We present the case of a 32-year-old woman diagnosed of PV in December 2018 and followed up at Guy’s Hospital, who was on ropeginterferon alpha-2b during pregnancy, with adequate control and no complications.
Results
In December 2018, at the age of 29, she presented with numbness and intermittent paraesthesia in the right side of her face and body. She had had previous history of skin rash and bilateral conjunctival congestion. She had no history of thrombotic events. Neurological causes of her hyperviscosity syndrome were ruled out. Her leukocyte count was 25.2 ×109/L (neutrophils 22.2 ×109/L), with haemoglobin (Hb) of 175 g/L, haematocrit (Hct) of 0.57 and platelets of 859 ×109/L. Abdominal ultrasound scan showed splenomegaly (16 cm). JAK2 V617F mutation was detected and PV, with bone marrow (BM) features of panmyelosis, no immature precursors and grade 1 fibrosis, was diagnosed according to WHO criteria.
She had several venesections and started aspirin 150 mg/day. Due to myeloproliferative features, treatment with peginterferon alfa-2a (Pegasys®), 90 mcg weekly, was added, with later increases up to 225 mcg weekly.
As she had no major response to peginterferon alfa-2a, with persistent high leukocyte and platelet counts (leukocytes 23.8 ×109/L, neutrophils 21.2 ×109/L, Hb 107 g/L, Hct 0.38 and platelets 926 ×109/L), teardrop cells in the blood film and enlarged spleen (23 cm), in February 2020 another BM was performed, excluding progression to myelofibrosis. During this time she had the desire to concieve, but could not become pregnant. She was then switched to ropeginterferon alfa-2b, 350 mcg every other week.
In March 2020 she conceived, continuing ropeginterferon alfa-2b, as well as thromboprophylaxis with aspirin and enoxaparin 40 mg/day. She had several venesections during pregnancy, in order to maintain Hct <0.38 per BSH guidelines. She underwent local follow up with no special symptoms or signs of abnormal fetal development. Ultrasound scan in the third trimester demonstrated stability in spleen size.
The baby was delivered on 14th December 2020, at 38 weeks of gestation (weight 2.8 kg). The patient’s most recent blood control in January 2021, after delivery, showed a leukocyte count of 19.1 ×109/L (neutrophils 15.7 ×109/L), Hb of 119 g/L, Hct of 0.42 and platelet count of 855 ×109/L.

Conclusion
To our knowledge, this is the first case in which ropeginterferon alfa-2b is used in a pregnant PV patient and also the first reported case where a patient with inadequate disease control with peginterferon alfa-2a attained sufficient response with ropeginterferon alfa-2b.
An informed consent has been obtained from the patient to write this abstract.
Keyword(s): Myeloproliferative disorder, Pegylated Interferon, Polycythemia vera, Pregnancy


