
Contributions
Abstract: PB1712
Type: Publication Only
Session title: Myeloproliferative neoplasms - Clinical
Background
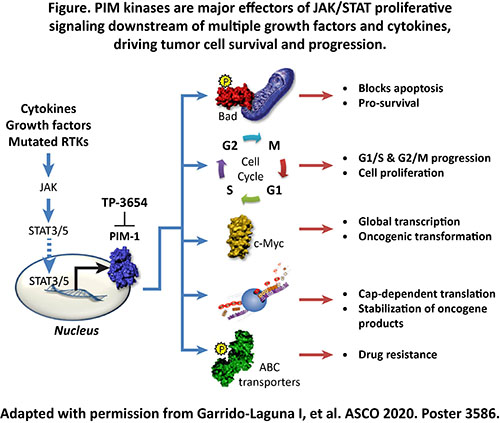
Myelofibrosis (MF) is characterized by bone marrow fibrosis (BMF), ineffective hematopoiesis, splenomegaly, and debilitating symptoms. An activating Janus kinase 2 (JAK2) mutation (JAK2 V617F) has been frequently observed in MF. Ruxolitinib and fedratinib, approved JAK inhibitors, have been shown to reduce splenomegaly and improve constitutional symptoms in MF patients, but with a modest reduction of BMF. Patients (pts) with MF who progressed on, are intolerant to, or are ineligible for JAK inhibitors have limited treatment options. Proviral integration site of Moloney murine leukemia virus (PIM) serine/threonine kinases are downstream targets of the JAK/STAT signaling pathway (Figure). Expression of PIM1 is significantly elevated in MF primary hematopoietic cells. Therefore, PIM kinase is a potential therapeutic target for MF. TP-3654, an oral, small molecule, investigational PIM kinase inhibitor, has been shown to inhibit proliferation and increase apoptosis in murine and human hematopoietic cells expressing JAK2 V617F mutation. TP-3654, alone and in combination with ruxolitinib, improves leukocyte counts, reduces spleen size, and reduces BMF in a JAK2 V617F murine MF model (Nath D, et al. Blood. 2018;132(suppl 1):54).
Aims
To evaluate TP-3654 monotherapy in pts with MF.
Methods
This phase I, multicenter, open-label, dose-escalation study is evaluating TP-3654 monotherapy in pts with MF who previously failed or are ineligible to receive a JAK inhibitor (ruxolitinib or fedratinib) (NCT04176198). Primary objectives are to determine the maximum tolerated dose (MTD) and/or to identify the recommended phase II dose (RP2D) of TP-3654 monotherapy. Secondary objectives are to assess preliminary activity (spleen volume reduction, symptom improvement, and BMF reduction), determine QT interval changes, and establish the pharmacokinetic profile. Exploratory objectives are to study pharmacodynamic markers in peripheral blood and bone marrow (BM) biopsy samples. Eligible pts have primary or secondary MF (post-polycythemia vera-MF/post-essential thrombocythemia-MF); intermediate-2/high-risk MF by the Dynamic International Prognostic Scoring System (DIPSS); previously failed or are ineligible to receive treatment with a JAK inhibitor; grade ≥2 MF, as confirmed by BM biopsy ≤12 weeks prior to screening; have a platelet count ≥50x109/L, absolute neutrophil count ≥1x109/L, hemoglobin level ≥8 g/L, peripheral blood blast counts <10%, ECOG performance status ≤2, life expectancy ≥3 months, and adequate renal and hepatic function; have spleen length ≥5 cm by palpation or spleen volume ≥450 cm3 by CT/MRI, and show ≥2 measurable symptoms per the MF Symptom Assessment Form, version 4.0. Pts must not have had splenic irradiation ≤6 months prior to screening; have acute myeloid leukemia or myelodysplastic syndrome; or had prior stem cell transplantation (SCT) or be eligible for allogeneic BM or SCT. Enrollment of approximately 50 pts with MF is planned. Pts will receive oral TP-3654. Dose escalation will be performed using a Bayesian Logistic Regression Model (BLRM) with Escalation with Overdose Control (EWOC). Adverse events occurring during the first cycle will be considered in the determination of the MTD. Dose escalation will continue until identification of the MTD and/or a suitable RP2D. This study is currently recruiting pts. Informed consent is being obtained for each participant/potential participant in this trial, in accordance with the International Conference on Harmonization-Good Clinical Practice.
Results
-
Conclusion
-
Keyword(s): Kinase inhibitor, Myelofibrosis, Phase I, Pim-1
Abstract: PB1712
Type: Publication Only
Session title: Myeloproliferative neoplasms - Clinical
Background
Myelofibrosis (MF) is characterized by bone marrow fibrosis (BMF), ineffective hematopoiesis, splenomegaly, and debilitating symptoms. An activating Janus kinase 2 (JAK2) mutation (JAK2 V617F) has been frequently observed in MF. Ruxolitinib and fedratinib, approved JAK inhibitors, have been shown to reduce splenomegaly and improve constitutional symptoms in MF patients, but with a modest reduction of BMF. Patients (pts) with MF who progressed on, are intolerant to, or are ineligible for JAK inhibitors have limited treatment options. Proviral integration site of Moloney murine leukemia virus (PIM) serine/threonine kinases are downstream targets of the JAK/STAT signaling pathway (Figure). Expression of PIM1 is significantly elevated in MF primary hematopoietic cells. Therefore, PIM kinase is a potential therapeutic target for MF. TP-3654, an oral, small molecule, investigational PIM kinase inhibitor, has been shown to inhibit proliferation and increase apoptosis in murine and human hematopoietic cells expressing JAK2 V617F mutation. TP-3654, alone and in combination with ruxolitinib, improves leukocyte counts, reduces spleen size, and reduces BMF in a JAK2 V617F murine MF model (Nath D, et al. Blood. 2018;132(suppl 1):54).
Aims
To evaluate TP-3654 monotherapy in pts with MF.
Methods
This phase I, multicenter, open-label, dose-escalation study is evaluating TP-3654 monotherapy in pts with MF who previously failed or are ineligible to receive a JAK inhibitor (ruxolitinib or fedratinib) (NCT04176198). Primary objectives are to determine the maximum tolerated dose (MTD) and/or to identify the recommended phase II dose (RP2D) of TP-3654 monotherapy. Secondary objectives are to assess preliminary activity (spleen volume reduction, symptom improvement, and BMF reduction), determine QT interval changes, and establish the pharmacokinetic profile. Exploratory objectives are to study pharmacodynamic markers in peripheral blood and bone marrow (BM) biopsy samples. Eligible pts have primary or secondary MF (post-polycythemia vera-MF/post-essential thrombocythemia-MF); intermediate-2/high-risk MF by the Dynamic International Prognostic Scoring System (DIPSS); previously failed or are ineligible to receive treatment with a JAK inhibitor; grade ≥2 MF, as confirmed by BM biopsy ≤12 weeks prior to screening; have a platelet count ≥50x109/L, absolute neutrophil count ≥1x109/L, hemoglobin level ≥8 g/L, peripheral blood blast counts <10%, ECOG performance status ≤2, life expectancy ≥3 months, and adequate renal and hepatic function; have spleen length ≥5 cm by palpation or spleen volume ≥450 cm3 by CT/MRI, and show ≥2 measurable symptoms per the MF Symptom Assessment Form, version 4.0. Pts must not have had splenic irradiation ≤6 months prior to screening; have acute myeloid leukemia or myelodysplastic syndrome; or had prior stem cell transplantation (SCT) or be eligible for allogeneic BM or SCT. Enrollment of approximately 50 pts with MF is planned. Pts will receive oral TP-3654. Dose escalation will be performed using a Bayesian Logistic Regression Model (BLRM) with Escalation with Overdose Control (EWOC). Adverse events occurring during the first cycle will be considered in the determination of the MTD. Dose escalation will continue until identification of the MTD and/or a suitable RP2D. This study is currently recruiting pts. Informed consent is being obtained for each participant/potential participant in this trial, in accordance with the International Conference on Harmonization-Good Clinical Practice.
Results
-

Conclusion
-
Keyword(s): Kinase inhibitor, Myelofibrosis, Phase I, Pim-1


