
Contributions
Abstract: PB1709
Type: Publication Only
Session title: Myeloproliferative neoplasms - Clinical
Background
BLU-263 is an investigational, potent, and selective oral small molecule inhibitor of KIT with sub-nanomolar potency against D816V-mutant KIT. Systemic mastocytosis is driven by the KIT D816V mutation in approximately 95% of cases and is vulnerable to KIT D816V inhibition. BLU-263 was highly active in a murine model of mastocytosis and was well tolerated with minimal central nervous system penetration in preclinical species.
Aims
Here we report the safety, tolerability, and pharmacokinetics (PK) of BLU-263 in an ongoing Phase 1, randomized, double-blind, placebo-controlled single ascending dose (SAD) and multiple ascending dose (MAD) study.
Methods
In the SAD portion of the study, cohorts of eight normal healthy volunteers aged ≥19 years received either oral placebo (n=2/cohort) or single oral doses of BLU‑263 (n=6/cohort) of 15, 25, 50, 100, or 200 mg. In the MAD portion of the study, normal healthy volunteer cohorts received either oral placebo or BLU-263 at 25, 50, or 100 mg once daily (QD) on each of 10 consecutive days. Blood plasma samples were collected for PK analyses at pre-dose and 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 24, 48, 72, and 96 hours post-dose in SAD cohorts, and the same post-dose time points after dosing on days 1 and 10 in the MAD cohorts. Safety was assessed according to incidence and severity of adverse events (AEs) in recipients of BLU-263 as compared with placebo.
Results
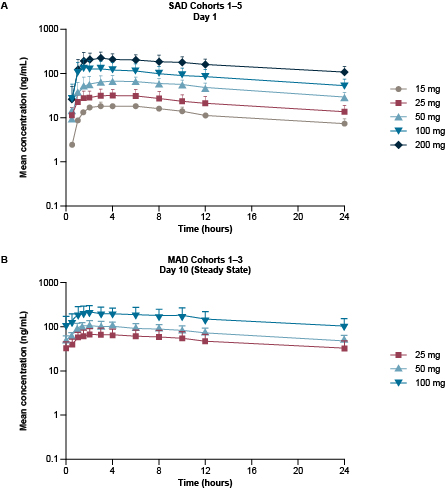
BLU-263 was well tolerated across the five BLU-263 SAD cohorts and three MAD cohorts. Only Grade 1 AEs were reported, and most were assessed as not related to the study drug by the investigator. These AEs included pain at the blood-sampling site, headache, dry/itchy skin, nausea, and stomachache. No serious AEs were reported, and there were no clinically relevant findings in laboratory, electrocardiogram, or vital signs parameters. After administration of BLU-263 at single doses, the median time to maximum concentration ranged from 1.5 to 6 hours post-dose. The mean half-life of BLU‑263 ranged from 20 hours to 28 hours, indicating that steady-state would be reached by Day 7, supporting QD dosing. After 10 days of repeated oral administration of 25 to 100 mg BLU‑263, the geometric mean accumulation ratio for area under the curve ranged from 1.6 to 1.8. The geometric mean apparent volume of distribution ranged from 753 to 973 L, indicating wide tissue distribution. Overall, a dose-proportional increase in systemic exposure to BLU-263 was observed across the SAD and MAD cohorts (Figure).
Conclusion
BLU-263, a next-generation investigational KIT inhibitor, was well tolerated across all SAD and MAD cohorts in normal healthy volunteers. The PK of BLU-263 were linear across the dose ranges in SAD and MAD cohorts and support QD dosing. These data support the continued development of BLU-263 in patients with systemic mastocytosis and other mast cell disorders.
Keyword(s): Kit, Systemic mastocytosis, Tyrosine kinase inhibitor
Abstract: PB1709
Type: Publication Only
Session title: Myeloproliferative neoplasms - Clinical
Background
BLU-263 is an investigational, potent, and selective oral small molecule inhibitor of KIT with sub-nanomolar potency against D816V-mutant KIT. Systemic mastocytosis is driven by the KIT D816V mutation in approximately 95% of cases and is vulnerable to KIT D816V inhibition. BLU-263 was highly active in a murine model of mastocytosis and was well tolerated with minimal central nervous system penetration in preclinical species.
Aims
Here we report the safety, tolerability, and pharmacokinetics (PK) of BLU-263 in an ongoing Phase 1, randomized, double-blind, placebo-controlled single ascending dose (SAD) and multiple ascending dose (MAD) study.
Methods
In the SAD portion of the study, cohorts of eight normal healthy volunteers aged ≥19 years received either oral placebo (n=2/cohort) or single oral doses of BLU‑263 (n=6/cohort) of 15, 25, 50, 100, or 200 mg. In the MAD portion of the study, normal healthy volunteer cohorts received either oral placebo or BLU-263 at 25, 50, or 100 mg once daily (QD) on each of 10 consecutive days. Blood plasma samples were collected for PK analyses at pre-dose and 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 24, 48, 72, and 96 hours post-dose in SAD cohorts, and the same post-dose time points after dosing on days 1 and 10 in the MAD cohorts. Safety was assessed according to incidence and severity of adverse events (AEs) in recipients of BLU-263 as compared with placebo.
Results
BLU-263 was well tolerated across the five BLU-263 SAD cohorts and three MAD cohorts. Only Grade 1 AEs were reported, and most were assessed as not related to the study drug by the investigator. These AEs included pain at the blood-sampling site, headache, dry/itchy skin, nausea, and stomachache. No serious AEs were reported, and there were no clinically relevant findings in laboratory, electrocardiogram, or vital signs parameters. After administration of BLU-263 at single doses, the median time to maximum concentration ranged from 1.5 to 6 hours post-dose. The mean half-life of BLU‑263 ranged from 20 hours to 28 hours, indicating that steady-state would be reached by Day 7, supporting QD dosing. After 10 days of repeated oral administration of 25 to 100 mg BLU‑263, the geometric mean accumulation ratio for area under the curve ranged from 1.6 to 1.8. The geometric mean apparent volume of distribution ranged from 753 to 973 L, indicating wide tissue distribution. Overall, a dose-proportional increase in systemic exposure to BLU-263 was observed across the SAD and MAD cohorts (Figure).

Conclusion
BLU-263, a next-generation investigational KIT inhibitor, was well tolerated across all SAD and MAD cohorts in normal healthy volunteers. The PK of BLU-263 were linear across the dose ranges in SAD and MAD cohorts and support QD dosing. These data support the continued development of BLU-263 in patients with systemic mastocytosis and other mast cell disorders.
Keyword(s): Kit, Systemic mastocytosis, Tyrosine kinase inhibitor


