
Contributions
Abstract: PB1707
Type: Publication Only
Session title: Myeloproliferative neoplasms - Clinical
Background
Polypharmacy is defined as the concurrent use of 5 or more medications/day by a single subject. As the lifespan increases worldwide, patients diagnosed with essential thrombocythemia (ET) are often exposed to polypharmacy due to age-related comorbidities and lifelong ET management.
Aims
Identify polypharmacy use and the risk of potential drug-drug (DDI)/food-drug interactions (FDI) in ET patients on risk-adapted therapy.
Methods
The electronic medical records of the Internal Medicine Clinic, Clinical Emergency Hospital of Bucharest, Bucharest, Romania, were searched from inception (available since August 2014) to February 2021. We included all ET patients who received risk-adapted therapy, had signed the written informed consent for participation in medical research and for whom information regarding prescribed medications was available. The study was approved by the hospital's ethics council (35552/22.10.2020). DDI/FDI were evaluated using an online drugs interaction checker.
Results
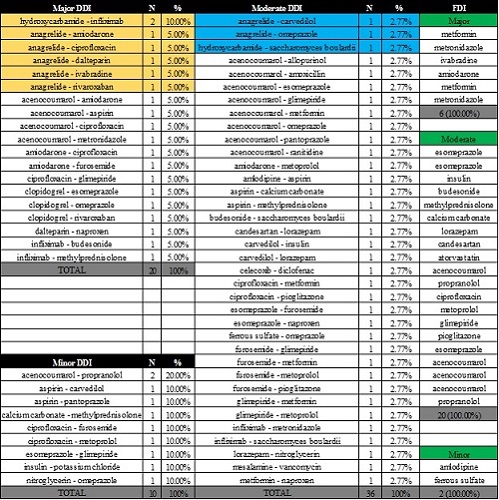
We identified 15 ET patients, median age 65 years, range 41-80 years, 60.0% females. Cytoreductive therapy was based predominantly on hydroxycarbamide (73.3%), anagrelide (13.3%) or their combination (13.4%). Due to thrombotic risk and associated comorbidities, most patients received antiplatelet drugs (acetylsalicylic acid 13.3%, clopidogrel 13.3%) or systemic anticoagulation alone (acenocoumarol 20.0%, dalteparin 13.3%) or their combination (acetylsalicylic acid + acenocoumarol 6.67%, clopidogrel + rivaroxaban 6.67%). Polypharmacy was detected in 66.67% of ET cases, all patients receiving at least 3 drugs/day. The mean number of prescribed drugs, DDI and FDI were 6.67±2.91 (range 3-12), 4.40±5.83 (range 0-24) and 1.87±1.82 (range 0-8). We recorded 66 DDI (20 major, 1.33±1.81/patient; 36 moderate, 2.40±3.44/patient; 10 minor, 0.67±0.87/patient) and 28 FDI (6 major, 0.40±0.61/patient; 20 moderate, 1.33±1.49/patient; 2 minor, 0.13±0.34/patient). Hydroxycarbamide led to 2 major DDI with infliximab and 1 moderate DDI with Saccharomyces boulardii. Anagrelide led to 5 major DDI with amiodarone, ciprofloxacin, dalteparin, ivabradine and rivaroxaban, and 2 moderate DDI with carvedilol and omeprazole. The main DDI and the drugs which led to FDI are reported in the attached tabel. The mean number of drugs prescribed correlated with the number of DDI (r=+0.887, P<0.001), major DDI (r=+0.743, P=0.002), moderate DDI (r=+0.834, P<0.001), minor DDI (r=+0.706, P=0.003), FDI (r=+0.735, P=0.002), moderate FDI (r=+0.731, P=0.002), but not with the number of major/minor FDI (P=0.207 and P=0.242, respectively). The number of DDI-per-patient also correlated with the number of FDI-per-patient (r=+0.879, P<0.001), major (r=+0.548, P=0.035) and moderate (r=+0.785, P=0.001) FDI, but not minor FDI (P=0.179). Surprisingly, there was no correlation between the age of ET patients and the number of drugs prescribed/DDI/FDI or major/moderate/minor FDI/DDI.
Conclusion
Risk-adapted therapy remains safe in ET, yet due to age-related comorbidities and increased risk of polypharmacy, these patients should be closely monitored to avoid DDI and FDI and reduce medication burden. To our knowledge, this is the first assessment of polypharmacy and DDI/FDI in ET. However, since there was no correlation of the number of drugs prescribed or of the DDI/FDI with the age of the participants, this might signal that polypharmacy is emerging as a public health issue irrespective of age and points out that the multidisciplinary team involved in the management of ET patients could also include clinical pharmacists/pharmacologists.
Keyword(s): Age, Anagrelide, Essential Thrombocytemia, Hydroxyurea
Abstract: PB1707
Type: Publication Only
Session title: Myeloproliferative neoplasms - Clinical
Background
Polypharmacy is defined as the concurrent use of 5 or more medications/day by a single subject. As the lifespan increases worldwide, patients diagnosed with essential thrombocythemia (ET) are often exposed to polypharmacy due to age-related comorbidities and lifelong ET management.
Aims
Identify polypharmacy use and the risk of potential drug-drug (DDI)/food-drug interactions (FDI) in ET patients on risk-adapted therapy.
Methods
The electronic medical records of the Internal Medicine Clinic, Clinical Emergency Hospital of Bucharest, Bucharest, Romania, were searched from inception (available since August 2014) to February 2021. We included all ET patients who received risk-adapted therapy, had signed the written informed consent for participation in medical research and for whom information regarding prescribed medications was available. The study was approved by the hospital's ethics council (35552/22.10.2020). DDI/FDI were evaluated using an online drugs interaction checker.
Results
We identified 15 ET patients, median age 65 years, range 41-80 years, 60.0% females. Cytoreductive therapy was based predominantly on hydroxycarbamide (73.3%), anagrelide (13.3%) or their combination (13.4%). Due to thrombotic risk and associated comorbidities, most patients received antiplatelet drugs (acetylsalicylic acid 13.3%, clopidogrel 13.3%) or systemic anticoagulation alone (acenocoumarol 20.0%, dalteparin 13.3%) or their combination (acetylsalicylic acid + acenocoumarol 6.67%, clopidogrel + rivaroxaban 6.67%). Polypharmacy was detected in 66.67% of ET cases, all patients receiving at least 3 drugs/day. The mean number of prescribed drugs, DDI and FDI were 6.67±2.91 (range 3-12), 4.40±5.83 (range 0-24) and 1.87±1.82 (range 0-8). We recorded 66 DDI (20 major, 1.33±1.81/patient; 36 moderate, 2.40±3.44/patient; 10 minor, 0.67±0.87/patient) and 28 FDI (6 major, 0.40±0.61/patient; 20 moderate, 1.33±1.49/patient; 2 minor, 0.13±0.34/patient). Hydroxycarbamide led to 2 major DDI with infliximab and 1 moderate DDI with Saccharomyces boulardii. Anagrelide led to 5 major DDI with amiodarone, ciprofloxacin, dalteparin, ivabradine and rivaroxaban, and 2 moderate DDI with carvedilol and omeprazole. The main DDI and the drugs which led to FDI are reported in the attached tabel. The mean number of drugs prescribed correlated with the number of DDI (r=+0.887, P<0.001), major DDI (r=+0.743, P=0.002), moderate DDI (r=+0.834, P<0.001), minor DDI (r=+0.706, P=0.003), FDI (r=+0.735, P=0.002), moderate FDI (r=+0.731, P=0.002), but not with the number of major/minor FDI (P=0.207 and P=0.242, respectively). The number of DDI-per-patient also correlated with the number of FDI-per-patient (r=+0.879, P<0.001), major (r=+0.548, P=0.035) and moderate (r=+0.785, P=0.001) FDI, but not minor FDI (P=0.179). Surprisingly, there was no correlation between the age of ET patients and the number of drugs prescribed/DDI/FDI or major/moderate/minor FDI/DDI.

Conclusion
Risk-adapted therapy remains safe in ET, yet due to age-related comorbidities and increased risk of polypharmacy, these patients should be closely monitored to avoid DDI and FDI and reduce medication burden. To our knowledge, this is the first assessment of polypharmacy and DDI/FDI in ET. However, since there was no correlation of the number of drugs prescribed or of the DDI/FDI with the age of the participants, this might signal that polypharmacy is emerging as a public health issue irrespective of age and points out that the multidisciplinary team involved in the management of ET patients could also include clinical pharmacists/pharmacologists.
Keyword(s): Age, Anagrelide, Essential Thrombocytemia, Hydroxyurea


