
Contributions
Abstract: PB1703
Type: Publication Only
Session title: Myeloproliferative neoplasms - Clinical
Background
The CD34+ cells in peripheral blood (PB) increased in myeloproliferative neoplasm (MPN) patients with myelofibrosis (MF) compared with MPN patients without MF, and were significantly correlated with disease duration, spleen volume, liver volume and risk classification. Further, the CD34+ cells in PB could be useful as a biomarker for prognosis and follow-up in the allo-SCT setting in MPN patients with MF.
Flow cytometric applications for CD34+ cell enumeration provide a rapid, quantitative, and reproducible method. However, significant site-to-site variation has been reported. Moreover, the variability associated with hematology-derived absolute counts is quite considerable. Single-platform flow cytometric absolute cell counting protocols have been shown to provide increased robustness of CD34+ enumeration. Enumeration of the cell populations in this study is obtained using an automated method for gating and analysis.
Aims
This study aimed to investigate the correlation between the frequency of CD34+ cells per CD45+ cells in PB (CD34+ cells/CD45+ cells) and MF progression, splenomegaly or cytokine levels.
Methods
We measured CD34+ cells/CD45+ cells of 19 healthy controls (HCs) and 52 MPN patients without ruxolitinib (Rux) treatment using BDTM Stem Cell Enumeration Kit®. The MPN patients with Rux treatment were excluded from this study because these patients had already been diagnosed MF before Rux treatment and Rux could affect the cytokine profiles and splenomegaly. The bone marrow biopsy was also performed at the same time as the PB sample collection. We also examined the concentration of 24 cytokines in the serum of 52 MPN patients using Human Luminex® Assays. Thereafter, we assessed the correlations between CD34+ cells/CD45+ cells and MF onset, splenomegaly or cytokine levels.
Results
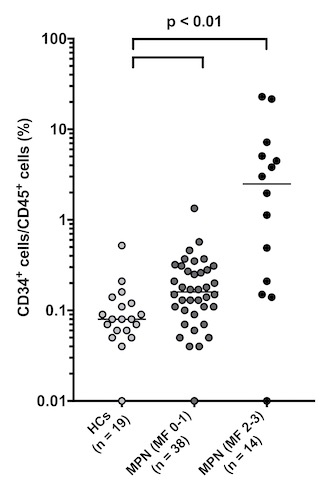
The median percentage of CD34+ cells/CD45+ cells of HCs (n = 19), MPN patients with MF grade 0-1 (n = 38) and MPN patients with MF grade 2-3 (n = 14) were 0.08%, 0.16%, and 2.49%, respectively. Compared with HCs and MPN patients with MF grade 0-1, CD34+ cells/CD45+ cells of MPN patients with MF grade 2-3 significantly increased (p < 0.01). Further, MPN patients with MF grade 2-3 had significantly higher CD34+ cells/CD45+ cells than MPN patients with MF grade 0-1 (p < 0.01). For the ROC curve, the sensitivity and specificity for classifying MF grade 2-3 were 71.4% and 94.7% with the cutoff set to > 0.5%. Moreover, the sensitivity for detecting MF progression of CD34+ cells/CD45+ cells (71.4%) was approximately two times higher than that of the blast emergence in hemogram (35.7%). On the other hand, the median percentage of CD34+ cells/CD45+ cells of MPN patients without splenomegaly (n = 41) and MPN patients with splenomegaly (n = 11) were 0.17% and 3.81%. MPN patients with splenomegaly had significantly higher CD34+ cells/CD45+ cells than those without splenomegaly (p < 0.01). For the ROC curve, the sensitivity and specificity for classifying splenomegaly onset were 72.7% and 95.1% with the cutoff set to > 1.0%. In addition, TNF-a, IL-8 and CXCL1 showed significant correlations with CD34+ cells/CD45+ cells (R2 = 0.538, 0.435 and 0.260, respectively).
Conclusion
In our study, CD34+ cells/CD45+ cells in PB elevated in MPN patients with MF grade 2-3, and was significantly correlated with MF grade, splenomegaly, TNF-a, IL-8 and CXCL1. It would be worth evaluating the efficacy of CD34+ cells/CD45+ cells as non-invasive markers of MF progression in a prospective study.
Keyword(s): Automation, CD34+ cells, Myelofibrosis, Myeloproliferative disorder
Abstract: PB1703
Type: Publication Only
Session title: Myeloproliferative neoplasms - Clinical
Background
The CD34+ cells in peripheral blood (PB) increased in myeloproliferative neoplasm (MPN) patients with myelofibrosis (MF) compared with MPN patients without MF, and were significantly correlated with disease duration, spleen volume, liver volume and risk classification. Further, the CD34+ cells in PB could be useful as a biomarker for prognosis and follow-up in the allo-SCT setting in MPN patients with MF.
Flow cytometric applications for CD34+ cell enumeration provide a rapid, quantitative, and reproducible method. However, significant site-to-site variation has been reported. Moreover, the variability associated with hematology-derived absolute counts is quite considerable. Single-platform flow cytometric absolute cell counting protocols have been shown to provide increased robustness of CD34+ enumeration. Enumeration of the cell populations in this study is obtained using an automated method for gating and analysis.
Aims
This study aimed to investigate the correlation between the frequency of CD34+ cells per CD45+ cells in PB (CD34+ cells/CD45+ cells) and MF progression, splenomegaly or cytokine levels.
Methods
We measured CD34+ cells/CD45+ cells of 19 healthy controls (HCs) and 52 MPN patients without ruxolitinib (Rux) treatment using BDTM Stem Cell Enumeration Kit®. The MPN patients with Rux treatment were excluded from this study because these patients had already been diagnosed MF before Rux treatment and Rux could affect the cytokine profiles and splenomegaly. The bone marrow biopsy was also performed at the same time as the PB sample collection. We also examined the concentration of 24 cytokines in the serum of 52 MPN patients using Human Luminex® Assays. Thereafter, we assessed the correlations between CD34+ cells/CD45+ cells and MF onset, splenomegaly or cytokine levels.
Results
The median percentage of CD34+ cells/CD45+ cells of HCs (n = 19), MPN patients with MF grade 0-1 (n = 38) and MPN patients with MF grade 2-3 (n = 14) were 0.08%, 0.16%, and 2.49%, respectively. Compared with HCs and MPN patients with MF grade 0-1, CD34+ cells/CD45+ cells of MPN patients with MF grade 2-3 significantly increased (p < 0.01). Further, MPN patients with MF grade 2-3 had significantly higher CD34+ cells/CD45+ cells than MPN patients with MF grade 0-1 (p < 0.01). For the ROC curve, the sensitivity and specificity for classifying MF grade 2-3 were 71.4% and 94.7% with the cutoff set to > 0.5%. Moreover, the sensitivity for detecting MF progression of CD34+ cells/CD45+ cells (71.4%) was approximately two times higher than that of the blast emergence in hemogram (35.7%). On the other hand, the median percentage of CD34+ cells/CD45+ cells of MPN patients without splenomegaly (n = 41) and MPN patients with splenomegaly (n = 11) were 0.17% and 3.81%. MPN patients with splenomegaly had significantly higher CD34+ cells/CD45+ cells than those without splenomegaly (p < 0.01). For the ROC curve, the sensitivity and specificity for classifying splenomegaly onset were 72.7% and 95.1% with the cutoff set to > 1.0%. In addition, TNF-a, IL-8 and CXCL1 showed significant correlations with CD34+ cells/CD45+ cells (R2 = 0.538, 0.435 and 0.260, respectively).

Conclusion
In our study, CD34+ cells/CD45+ cells in PB elevated in MPN patients with MF grade 2-3, and was significantly correlated with MF grade, splenomegaly, TNF-a, IL-8 and CXCL1. It would be worth evaluating the efficacy of CD34+ cells/CD45+ cells as non-invasive markers of MF progression in a prospective study.
Keyword(s): Automation, CD34+ cells, Myelofibrosis, Myeloproliferative disorder


