
Contributions
Abstract: PB1702
Type: Publication Only
Session title: Myeloproliferative neoplasms - Clinical
Background
Myeloproliferative neoplasm (MPN)-associated myelofibrosis (MF) is a clonal myeloid neoplasm characterized by defective bone marrow (BM) function, BM fibrosis, ineffective erythropoiesis, and increased risk of progression to acute myeloid leukemia (AML). Up to 60% of patients (pts) with MF present with anemia at diagnosis, and eventually all pts go on to develop anemia. Anemia associated with transfusion-dependent MF is associated with poor survival and there are limited treatment options. Luspatercept is a first and only erythroid maturation agent approved in both the EU and the USA for treatment of anemia in pts with β-thalassemia who require red blood cell (RBC) transfusions and in pts with lower-risk myelodysplastic syndromes with ring sideroblasts who failed erythropoiesis-stimulating agent (ESA) treatment and require RBC transfusions. In a preliminary analysis of a phase 2 trial evaluating luspatercept in pts with MF-associated anemia (NCT03194542), 27% of pts achieved at least one ≥12-week (wk) episode of RBC transfusion independence (TI) with luspatercept within the first 24 wks of treatment (Gerds et al. Blood 2020;136(suppl 1):47−48).
Aims
This trials-in-progress abstract details eligibility criteria and trial design of the currently enrolling phase 3 INDEPENDENCE trial.
Methods
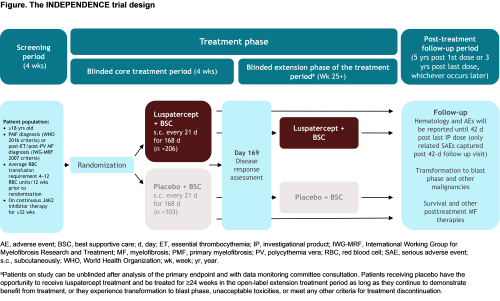
INDEPENDENCE is a phase 3, double-blind, randomized trial to evaluate the efficacy and safety of luspatercept versus placebo in pts with MPN-associated MF receiving concomitant JAK2 inhibitor therapy who require RBC transfusions. Eligible pts must be ≥18 years old, have a documented diagnosis of primary MF, post-essential thrombocythemia MF or post-polycythemia MF, require regular RBC transfusions (defined as 4−12 RBC units/12 wks prior to randomization), and receiving JAK2 inhibitor therapy for ≥32 wks (on stable daily dose for ≥16 wks) prior to randomization. Exclusion criteria include presence of anemia due to causes other than MPN-associated MF or JAK2 inhibitor therapy, and prior use of hydroxyurea, immunomodulatory compounds (pomalidomide, thalidomide), ESAs, androgenic steroids, or other drugs with potential effects on hematopoiesis ≤8 weeks before randomization. Following screening, ~309 pts will be randomized 2:1 to either luspatercept (starting at 1.33 mg/kg with titration up to 1.75 mg/kg) subcutaneously every 3 wks, or placebo (Figure). Best supportive care, including RBC transfusions, iron chelation therapy, antibiotic, antiviral, and/or antifungal therapy, is permitted in both treatment arms. Stratification factors include RBC transfusion burden and Dynamic International Prognostic Scoring System (DIPSS) score at baseline.
Results
Clinical benefit (CB) will be assessed after 24 wks of treatment, and is defined as RBC-TI for any consecutive ≥12-wk period during Wks 1–24 of study, a reduction in RBC transfusion burden by ≥50% and by ≥4 units for ≥12 wks during Wks 1–24, or an incomplete duration of response, defined as being free from RBC transfusions for ≥4 wks immediately up to the end of 24 wks. Pts achieving CB can continue luspatercept treatment as long as they continue to benefit, or until progression to AML, unacceptable toxicities, withdrawal of consent, or until meeting any other criteria for treatment discontinuation. Primary endpoint of the study is the proportion of pts who become RBC-transfusion free over any consecutive 12-wk period starting from randomization up to and including Wk 24.
Conclusion
The INDEPENDENCE trial is registered at EudraCT (2020-000607-36) and ClinicalTrials.gov (NCT04717414).
Keyword(s): Anemia, Clinical trial, Myelofibrosis, Myeloproliferative disorder
Abstract: PB1702
Type: Publication Only
Session title: Myeloproliferative neoplasms - Clinical
Background
Myeloproliferative neoplasm (MPN)-associated myelofibrosis (MF) is a clonal myeloid neoplasm characterized by defective bone marrow (BM) function, BM fibrosis, ineffective erythropoiesis, and increased risk of progression to acute myeloid leukemia (AML). Up to 60% of patients (pts) with MF present with anemia at diagnosis, and eventually all pts go on to develop anemia. Anemia associated with transfusion-dependent MF is associated with poor survival and there are limited treatment options. Luspatercept is a first and only erythroid maturation agent approved in both the EU and the USA for treatment of anemia in pts with β-thalassemia who require red blood cell (RBC) transfusions and in pts with lower-risk myelodysplastic syndromes with ring sideroblasts who failed erythropoiesis-stimulating agent (ESA) treatment and require RBC transfusions. In a preliminary analysis of a phase 2 trial evaluating luspatercept in pts with MF-associated anemia (NCT03194542), 27% of pts achieved at least one ≥12-week (wk) episode of RBC transfusion independence (TI) with luspatercept within the first 24 wks of treatment (Gerds et al. Blood 2020;136(suppl 1):47−48).
Aims
This trials-in-progress abstract details eligibility criteria and trial design of the currently enrolling phase 3 INDEPENDENCE trial.
Methods
INDEPENDENCE is a phase 3, double-blind, randomized trial to evaluate the efficacy and safety of luspatercept versus placebo in pts with MPN-associated MF receiving concomitant JAK2 inhibitor therapy who require RBC transfusions. Eligible pts must be ≥18 years old, have a documented diagnosis of primary MF, post-essential thrombocythemia MF or post-polycythemia MF, require regular RBC transfusions (defined as 4−12 RBC units/12 wks prior to randomization), and receiving JAK2 inhibitor therapy for ≥32 wks (on stable daily dose for ≥16 wks) prior to randomization. Exclusion criteria include presence of anemia due to causes other than MPN-associated MF or JAK2 inhibitor therapy, and prior use of hydroxyurea, immunomodulatory compounds (pomalidomide, thalidomide), ESAs, androgenic steroids, or other drugs with potential effects on hematopoiesis ≤8 weeks before randomization. Following screening, ~309 pts will be randomized 2:1 to either luspatercept (starting at 1.33 mg/kg with titration up to 1.75 mg/kg) subcutaneously every 3 wks, or placebo (Figure). Best supportive care, including RBC transfusions, iron chelation therapy, antibiotic, antiviral, and/or antifungal therapy, is permitted in both treatment arms. Stratification factors include RBC transfusion burden and Dynamic International Prognostic Scoring System (DIPSS) score at baseline.
Results
Clinical benefit (CB) will be assessed after 24 wks of treatment, and is defined as RBC-TI for any consecutive ≥12-wk period during Wks 1–24 of study, a reduction in RBC transfusion burden by ≥50% and by ≥4 units for ≥12 wks during Wks 1–24, or an incomplete duration of response, defined as being free from RBC transfusions for ≥4 wks immediately up to the end of 24 wks. Pts achieving CB can continue luspatercept treatment as long as they continue to benefit, or until progression to AML, unacceptable toxicities, withdrawal of consent, or until meeting any other criteria for treatment discontinuation. Primary endpoint of the study is the proportion of pts who become RBC-transfusion free over any consecutive 12-wk period starting from randomization up to and including Wk 24.

Conclusion
The INDEPENDENCE trial is registered at EudraCT (2020-000607-36) and ClinicalTrials.gov (NCT04717414).
Keyword(s): Anemia, Clinical trial, Myelofibrosis, Myeloproliferative disorder


