
Contributions
Abstract: PB1696
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
The organ damage at the time of AL amyloidosis diagnosis infers a high morbi-mortality and a poor prognosis. Most patients have some degree of cardiac involvement and the stringent selection criteria for ASCT exclude many of them despite an appropriate age, making upfront ASCT not a suitable option. The novel agent-based schemes (Palladini et al. Ash publications Blood 2020) with an excellent toxicity profile and efficacy may allow to undergo ASCT due to a better hematologic and organic response.
Aims
To describe our experience in patients treated in our center who are not eligible for ASCT (Kumar et al. J. Clin. Oncol. 2012) with an in induction treatment based on immunomodulator (IMID) and proteasome inhibitor (PI), with Daratumumab in one, allowing them to undergo ASCT later.
Methods
Descriptive and retrospective analysis of four cases of AL Amyloidosis, diagnosed and treated in our center between 2010 and 2020.
Results
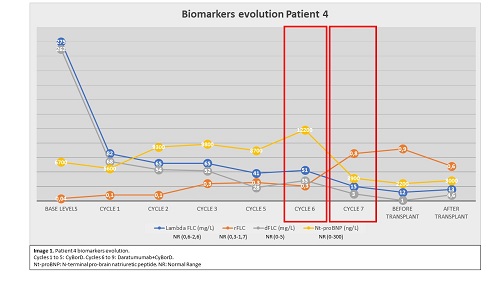
Baseline characteristics are described in Table 1A. The induction treatment, hematologic and organ response and the patient´s evolution are presented in table 1B. Patients 1, 2 and 4 presented severe cardiac damage and patient 3 has subacute liver failure (bilirubin 30 mg/dL). All the patients had a Mayo Clinic 2012 stage II or superior, so their survival prognosis was less than 1 year. After the induction treatment, we could perform an ASCT in all patients. Image 1 shows the evolution of the free light chains (FLC) of patient 4 with severe cardiac damage, and we present how the improvement of the hematologic response (to get dFLC less than 10 mg/L), allowed the organ response, and she could undergo a transplant. With a median follow-up of 23,5 months, all patients are currently alive and maintain a hematologic and organ response.
Tabla 1A. Patient | 1 | 2 | 3 | 4 |
Age (years)/Gender | 61/Female | 47/Female | 54/Male | 49/Female |
rFLC/dFLC (mg/L) | 0.1/81.8 | 0.092/- | 40/480 | 0.04/262.5 |
Creatinine (mg/dL)/24h urine protein (mg/dL) | 0.61/126 | -/70 | 2.5/570 | 0.65/3054 |
Nt-proBNP (ng/L)/TnT (ng/L) | 2236/60 | 10055/normal | 8231/50 | 6700/47.2 |
ECOG/NYHA | 3/III | 3/III | 3/I | 3/III |
LVEF (%)/thickness ventricular wall (mm) | 55/16 | 45/14 | 65/17 | 45/17 |
Bilirrubine (mg/dL) | 0.4 | - | 29.5 | 0.9 |
Mayo Stage 2012 | III | II | III | IIIB |
Table 1B. Patient | 1 | 2 | 3 | 4 |
Induction treatment (nº cycles)/months to best response | CyBorD (3)/7 | Lenalidomide-Melphalan-Dexamethasone (9)/9 | CyBorD (8)/1 | CyBorD (5) Daratumumab-CyBorD (4)/3 |
Hem./Organ response pre-ASCT | PR/stable heart disease*2 | CR/response heart disease * | CR/response liver disease*1 | Stringent CR/response heart disease* |
ECOG/NYHA pre-ASCT | 1/I | 1/I | 1/I | 1/I |
Timing of ASCT from diagnosis (months) | 5 | 13 | 9 | 13 |
Conditioning regimen/Post-ASCT complications | Mel-140/Engrafment Syndrome (ES), mild Congestive Heart Failure (CHF) | Mel-140/Mild CHF | Mel-200/ES | Mel-200/- |
Hem./ organ response nowadays | Stringent CR/CR | Stringent CR/CR | CR/CR | Stringent CR/- |
Follow-up time (months) | 24 | 132 | 23 | 18 |
*Response heart disease: decrease>30% of Nt-proBNP from the value at diagnosis; *1 Liver response: decrease>50% of the initial FA level; *2 Stable heart disease: Nt-proBNP and Tn without significative changes
Conclusion
In our experience, patients with the diagnosis of AL amyloidosis and severe organ damage who underwent a ASCT after an induction therapy using new agents outlived their initial poor prognosis.
Keyword(s): AL amyloidosis, Autologous hematopoietic stem cell transplantation, Induction
Abstract: PB1696
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
The organ damage at the time of AL amyloidosis diagnosis infers a high morbi-mortality and a poor prognosis. Most patients have some degree of cardiac involvement and the stringent selection criteria for ASCT exclude many of them despite an appropriate age, making upfront ASCT not a suitable option. The novel agent-based schemes (Palladini et al. Ash publications Blood 2020) with an excellent toxicity profile and efficacy may allow to undergo ASCT due to a better hematologic and organic response.
Aims
To describe our experience in patients treated in our center who are not eligible for ASCT (Kumar et al. J. Clin. Oncol. 2012) with an in induction treatment based on immunomodulator (IMID) and proteasome inhibitor (PI), with Daratumumab in one, allowing them to undergo ASCT later.
Methods
Descriptive and retrospective analysis of four cases of AL Amyloidosis, diagnosed and treated in our center between 2010 and 2020.
Results
Baseline characteristics are described in Table 1A. The induction treatment, hematologic and organ response and the patient´s evolution are presented in table 1B. Patients 1, 2 and 4 presented severe cardiac damage and patient 3 has subacute liver failure (bilirubin 30 mg/dL). All the patients had a Mayo Clinic 2012 stage II or superior, so their survival prognosis was less than 1 year. After the induction treatment, we could perform an ASCT in all patients. Image 1 shows the evolution of the free light chains (FLC) of patient 4 with severe cardiac damage, and we present how the improvement of the hematologic response (to get dFLC less than 10 mg/L), allowed the organ response, and she could undergo a transplant. With a median follow-up of 23,5 months, all patients are currently alive and maintain a hematologic and organ response.
Tabla 1A. Patient | 1 | 2 | 3 | 4 |
Age (years)/Gender | 61/Female | 47/Female | 54/Male | 49/Female |
rFLC/dFLC (mg/L) | 0.1/81.8 | 0.092/- | 40/480 | 0.04/262.5 |
Creatinine (mg/dL)/24h urine protein (mg/dL) | 0.61/126 | -/70 | 2.5/570 | 0.65/3054 |
Nt-proBNP (ng/L)/TnT (ng/L) | 2236/60 | 10055/normal | 8231/50 | 6700/47.2 |
ECOG/NYHA | 3/III | 3/III | 3/I | 3/III |
LVEF (%)/thickness ventricular wall (mm) | 55/16 | 45/14 | 65/17 | 45/17 |
Bilirrubine (mg/dL) | 0.4 | - | 29.5 | 0.9 |
Mayo Stage 2012 | III | II | III | IIIB |
Table 1B. Patient | 1 | 2 | 3 | 4 |
Induction treatment (nº cycles)/months to best response | CyBorD (3)/7 | Lenalidomide-Melphalan-Dexamethasone (9)/9 | CyBorD (8)/1 | CyBorD (5) Daratumumab-CyBorD (4)/3 |
Hem./Organ response pre-ASCT | PR/stable heart disease*2 | CR/response heart disease * | CR/response liver disease*1 | Stringent CR/response heart disease* |
ECOG/NYHA pre-ASCT | 1/I | 1/I | 1/I | 1/I |
Timing of ASCT from diagnosis (months) | 5 | 13 | 9 | 13 |
Conditioning regimen/Post-ASCT complications | Mel-140/Engrafment Syndrome (ES), mild Congestive Heart Failure (CHF) | Mel-140/Mild CHF | Mel-200/ES | Mel-200/- |
Hem./ organ response nowadays | Stringent CR/CR | Stringent CR/CR | CR/CR | Stringent CR/- |
Follow-up time (months) | 24 | 132 | 23 | 18 |
*Response heart disease: decrease>30% of Nt-proBNP from the value at diagnosis; *1 Liver response: decrease>50% of the initial FA level; *2 Stable heart disease: Nt-proBNP and Tn without significative changes

Conclusion
In our experience, patients with the diagnosis of AL amyloidosis and severe organ damage who underwent a ASCT after an induction therapy using new agents outlived their initial poor prognosis.
Keyword(s): AL amyloidosis, Autologous hematopoietic stem cell transplantation, Induction


