
Contributions
Abstract: PB1694
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Daratumumab, an anti-CD38 monoclonal antibody, was approved in May 2016 by the European Medicines Agency (EMA) for relapsed/refractory multiple myeloma (RRMM) patients whose previous therapies included a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD). In June 2020, subcutaneous (SC) daratumumab gained EMA approval.
Aims
To assess clinical characteristics, response rates and toxicity profile of RRMM patients treated with daratumumab monotherapy in 2 European centers.
Methods
This two-center retrospective study included 25 RRMM patients who started daratumumab monotherapy between 05/2018 and 09/2020 at Guy’s Hospital (London) or Hospital Príncipe de Asturias (Madrid). Patients' data were reviewed from electronic records. Patients who completed ≥1 cycles were included in response analysis. Kaplan-Meier was used for survival outcomes.
Results
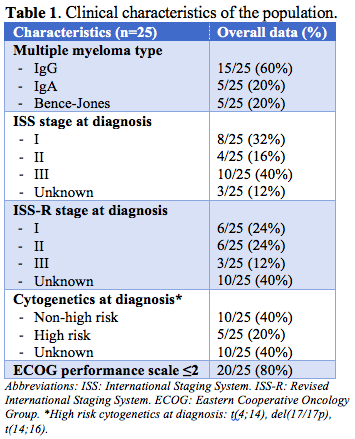
Median age at the start of daratumumab was 65 (range 47-91); 11 (44%) patients were male. Clinical characteristics are summarised in Table 1. All patients had previous exposure to a PI (bortezomib 100%, ixazomib 48%, carfilzomib 8%) and an IMiD (lenalidomide 100%, thalidomide 76%, pomalidomide 16%). Two (8%) were bortezomib-refractory, 1 (4%) was lenalidomide-refractory; another (4%) patient was refractory to both VTd (bortezomib, thalidomide, dexamethasone) and IRd (ixazomib, lenalidomide, dexamethasone) therapies. Twelve (50%) patients had undergone autologous stem cell transplant. Six (25%) had been included in a clinical trial (with no anti-CD38).
All patients had progression before starting daratumumab, with extramedullary disease in 4 (16%) patients. Median treatment duration was 4.3 months (m) (0.7-22.7) and median number of doses given was 28 (3-35). Seventeen (68%) patients discontinued therapy: 12 (70.6%) due to progressive disease and 5 (29.4%) died. Two deaths were due to refractory myeloma, 2 as a result of bleeding complications with thrombocytopenia grade≥3 and 1 sepsis-related.
At median follow-up of 8.7 months (0.7-23), 23 patients completed ≥1 cycles. Overall response rate (ORR) was 39.1% (9/23), with very good partial response (VGPR) in 17.4% (4/23). Median time to partial response (PR) was 2.3 m (2-3.6). Median progression-free survival (PFS) is 5.5 m, with longer PFS associated with completion of ≥1 cycle (median 6.5 months) and with deeper response (VGPR vs. PR or less, 15.3 vs. 3.9 m (p 0.012)). Median overall survival (OS) is 15.5 m.
Administration was SC in 4 (16%) patients and was converted from intravenous (IV) to SC in 4 (16%) others. First-dose infusion related reactions (IRR) occurred in 9 (36%) patients; 8/9 (88.9%) were associated with IV daratumumab and 3 were classified as grade ≥3 (including hypertension, fevers, desaturation and vomiting).
Common (≥15%) AEs were anaemia (92%), lymphopenia (80%), thrombocytopenia (72%), neutropenia (32%) and infections in 11 (44%), the majority being non-severe respiratory infections (45.4%).
Grade ≥3 AEs were found in 13 (52%) patients. Grade ≥3 thrombocytopenia was seen in 5 (20%), anaemia in 7 (28%), lymphopenia in 7 (28%) and neutropenia in 1 (4%). Grade ≥3 new proven infections after daratumumab start happened in 4/13 (30.8%) patients: sepsis related to Gram negative bacteriaemia leading to death, sepsis of unknown origin, pneumonia and urinary tract infection.
Conclusion
In summary, our experience with daratumumab monotherapy demonstrates encouraging response rates in heavily pretreated patients, similar to those reported in a pooled analysis of GEN501 and SIRIUS phase 1-2 trials. It shows an acceptable safety profile.
Keyword(s): CD38, Monoclonal antibody, Multiple myeloma, Refractory
Abstract: PB1694
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Daratumumab, an anti-CD38 monoclonal antibody, was approved in May 2016 by the European Medicines Agency (EMA) for relapsed/refractory multiple myeloma (RRMM) patients whose previous therapies included a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD). In June 2020, subcutaneous (SC) daratumumab gained EMA approval.
Aims
To assess clinical characteristics, response rates and toxicity profile of RRMM patients treated with daratumumab monotherapy in 2 European centers.
Methods
This two-center retrospective study included 25 RRMM patients who started daratumumab monotherapy between 05/2018 and 09/2020 at Guy’s Hospital (London) or Hospital Príncipe de Asturias (Madrid). Patients' data were reviewed from electronic records. Patients who completed ≥1 cycles were included in response analysis. Kaplan-Meier was used for survival outcomes.
Results
Median age at the start of daratumumab was 65 (range 47-91); 11 (44%) patients were male. Clinical characteristics are summarised in Table 1. All patients had previous exposure to a PI (bortezomib 100%, ixazomib 48%, carfilzomib 8%) and an IMiD (lenalidomide 100%, thalidomide 76%, pomalidomide 16%). Two (8%) were bortezomib-refractory, 1 (4%) was lenalidomide-refractory; another (4%) patient was refractory to both VTd (bortezomib, thalidomide, dexamethasone) and IRd (ixazomib, lenalidomide, dexamethasone) therapies. Twelve (50%) patients had undergone autologous stem cell transplant. Six (25%) had been included in a clinical trial (with no anti-CD38).
All patients had progression before starting daratumumab, with extramedullary disease in 4 (16%) patients. Median treatment duration was 4.3 months (m) (0.7-22.7) and median number of doses given was 28 (3-35). Seventeen (68%) patients discontinued therapy: 12 (70.6%) due to progressive disease and 5 (29.4%) died. Two deaths were due to refractory myeloma, 2 as a result of bleeding complications with thrombocytopenia grade≥3 and 1 sepsis-related.
At median follow-up of 8.7 months (0.7-23), 23 patients completed ≥1 cycles. Overall response rate (ORR) was 39.1% (9/23), with very good partial response (VGPR) in 17.4% (4/23). Median time to partial response (PR) was 2.3 m (2-3.6). Median progression-free survival (PFS) is 5.5 m, with longer PFS associated with completion of ≥1 cycle (median 6.5 months) and with deeper response (VGPR vs. PR or less, 15.3 vs. 3.9 m (p 0.012)). Median overall survival (OS) is 15.5 m.
Administration was SC in 4 (16%) patients and was converted from intravenous (IV) to SC in 4 (16%) others. First-dose infusion related reactions (IRR) occurred in 9 (36%) patients; 8/9 (88.9%) were associated with IV daratumumab and 3 were classified as grade ≥3 (including hypertension, fevers, desaturation and vomiting).
Common (≥15%) AEs were anaemia (92%), lymphopenia (80%), thrombocytopenia (72%), neutropenia (32%) and infections in 11 (44%), the majority being non-severe respiratory infections (45.4%).
Grade ≥3 AEs were found in 13 (52%) patients. Grade ≥3 thrombocytopenia was seen in 5 (20%), anaemia in 7 (28%), lymphopenia in 7 (28%) and neutropenia in 1 (4%). Grade ≥3 new proven infections after daratumumab start happened in 4/13 (30.8%) patients: sepsis related to Gram negative bacteriaemia leading to death, sepsis of unknown origin, pneumonia and urinary tract infection.

Conclusion
In summary, our experience with daratumumab monotherapy demonstrates encouraging response rates in heavily pretreated patients, similar to those reported in a pooled analysis of GEN501 and SIRIUS phase 1-2 trials. It shows an acceptable safety profile.
Keyword(s): CD38, Monoclonal antibody, Multiple myeloma, Refractory


