Contributions
Abstract: PB1689
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Anti-CD38 monoclonal antibodies (Mab) used in treatment of multiple myeloma (MM) patients have high response rates both in combination with other drugs and in monotherapy. However, a proportion of patients will eventually be resistant or relapsed (R/R). Patients R/R to anti-CD38 Mab present dismal prognosis and constitute a group for which therapeutic needs are still not defined.
Aims
In this study we aimed to describe the clinical and biological features as well as outcome of daratumumab R/R MM patients.
Methods
This is a retrospective analysis of 54 MM patients R/R to Daratumumab. Refractoriness was defined as progression within the first two months of treatment. Clinical and analytic data were collected from medical history. We used chi-square test to compare categorical variables and the Kaplan-Meier method and log rank test for the survival analysis. We performed multivariable analysis using logistic regression. IBM SPSS (v25.0) was used for the statistical analysis.
Results
The median age was 66 years; R(36-85), 57.4% were female. The cohort received a median of 4 lines of treatment; R(1-12). 70.4% had been previously treated with lenalidomide, 81.1% with bortezomib and 64.8% with both. Daratumumab was used as a first line therapy in 7 patients (14.8%), and as third or later line in 32 (59.3%). The median time to daratumumab progression was 6 months; R(1-53). 18 patients (33.3%) were primary refractory to daratumumab and 21 patients (38%) achieved very good partial response (VGPR) or better response. The median overall survival (OS) was 20 months; R(0-73). The most frequent combinations were with lenalidomide (DRd; 11%), bortezomib (DVd; 16%), bortezomib and melphalan (D-VMP; 13%) and with carfilzomib (KDd; 27%). 26% received daratumumab in monotherapy or with corticosteroids.
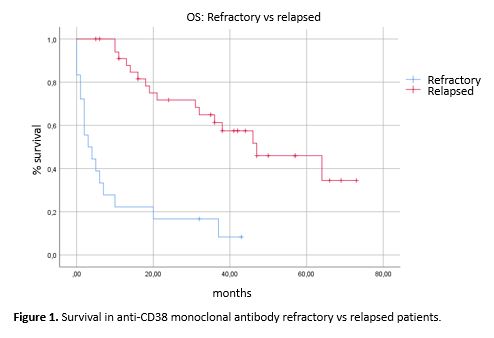
Only high cytogenetic risk (p=0,001) and treatment outside a clinical trial (p=0.003) were predictors of primary refractoriness to daratumumab. High cytogenetic risk remained statistically significant in the multivariable analysis (p=0.007). Patients with primary refractoriness to daratumumab had significantly shorter OS, with a median of 3 months vs 47 months in patients with eventual progression to Daratumumab (p=0,0001), as well as patients not treated within a clinical trial (p=0.0001) and patients treated in monotherapy (p=0.006). Interestingly, Daratumumab-resistant patients had longer OS when treated with regimens containing pomalidomide (median survival of 13 vs 46 months; p=0.021).
Resistance to daratumumab as a first line treatment was observed in 7 patients. The median OS in this group was 52 months and the median time of response to daratumumab was 12 months with 1 patient presenting refractoriness. All of them were treated within a clinical trial and none had high risk cytogenetics.
Conclusion
Patients R/R to CD-38 targeted monoclonal antibodies present dismal prognosis, with a median OS of 20 months. Only high cytogenetic risk was predictive of primary refractoriness according to the literature. Patients who received anti-CD38 Mab as a first line treatment had longer responses and OS, although the differences did not reach statistical significance, suggesting that even in this poor prognosis setting, patients benefit from using potent treatments as a first line. Particularly, the cohort treated with pomalidomide after daratumumab progression reached a longer OS, indicating that new drugs may represent an interesting approach for this subgroup of patients. Studies describing mechanisms of resistance to anti-cD38 Mab are essential to select the best option for each patient.
Keyword(s): CD38, Myeloma, Refractory, Relapse
Abstract: PB1689
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Anti-CD38 monoclonal antibodies (Mab) used in treatment of multiple myeloma (MM) patients have high response rates both in combination with other drugs and in monotherapy. However, a proportion of patients will eventually be resistant or relapsed (R/R). Patients R/R to anti-CD38 Mab present dismal prognosis and constitute a group for which therapeutic needs are still not defined.
Aims
In this study we aimed to describe the clinical and biological features as well as outcome of daratumumab R/R MM patients.
Methods
This is a retrospective analysis of 54 MM patients R/R to Daratumumab. Refractoriness was defined as progression within the first two months of treatment. Clinical and analytic data were collected from medical history. We used chi-square test to compare categorical variables and the Kaplan-Meier method and log rank test for the survival analysis. We performed multivariable analysis using logistic regression. IBM SPSS (v25.0) was used for the statistical analysis.
Results
The median age was 66 years; R(36-85), 57.4% were female. The cohort received a median of 4 lines of treatment; R(1-12). 70.4% had been previously treated with lenalidomide, 81.1% with bortezomib and 64.8% with both. Daratumumab was used as a first line therapy in 7 patients (14.8%), and as third or later line in 32 (59.3%). The median time to daratumumab progression was 6 months; R(1-53). 18 patients (33.3%) were primary refractory to daratumumab and 21 patients (38%) achieved very good partial response (VGPR) or better response. The median overall survival (OS) was 20 months; R(0-73). The most frequent combinations were with lenalidomide (DRd; 11%), bortezomib (DVd; 16%), bortezomib and melphalan (D-VMP; 13%) and with carfilzomib (KDd; 27%). 26% received daratumumab in monotherapy or with corticosteroids.
Only high cytogenetic risk (p=0,001) and treatment outside a clinical trial (p=0.003) were predictors of primary refractoriness to daratumumab. High cytogenetic risk remained statistically significant in the multivariable analysis (p=0.007). Patients with primary refractoriness to daratumumab had significantly shorter OS, with a median of 3 months vs 47 months in patients with eventual progression to Daratumumab (p=0,0001), as well as patients not treated within a clinical trial (p=0.0001) and patients treated in monotherapy (p=0.006). Interestingly, Daratumumab-resistant patients had longer OS when treated with regimens containing pomalidomide (median survival of 13 vs 46 months; p=0.021).
Resistance to daratumumab as a first line treatment was observed in 7 patients. The median OS in this group was 52 months and the median time of response to daratumumab was 12 months with 1 patient presenting refractoriness. All of them were treated within a clinical trial and none had high risk cytogenetics.
Conclusion
Patients R/R to CD-38 targeted monoclonal antibodies present dismal prognosis, with a median OS of 20 months. Only high cytogenetic risk was predictive of primary refractoriness according to the literature. Patients who received anti-CD38 Mab as a first line treatment had longer responses and OS, although the differences did not reach statistical significance, suggesting that even in this poor prognosis setting, patients benefit from using potent treatments as a first line. Particularly, the cohort treated with pomalidomide after daratumumab progression reached a longer OS, indicating that new drugs may represent an interesting approach for this subgroup of patients. Studies describing mechanisms of resistance to anti-cD38 Mab are essential to select the best option for each patient.
Keyword(s): CD38, Myeloma, Refractory, Relapse