
Contributions
Abstract: PB1684
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Multiple myeloma (MM) remains uncurable disease, with multiple relapses and clone evolution leading to refractoriness. However, recently significant therapeutical advancements were achieved leading to improvement of patient’s survival.
Aims
The aim of this analysis was to show real world data on treating multiple myeloma patients with relapsed/refractory (RR) disease with daratumumab.
Methods
We performed a retrospective analysis of outcomes of MM patients treated with daratumumab, in combination with bortezomib and dexamethasone (DVd) or lenalidomide and dexamethasone (DRd) in 13 Croatian hematology centers in the period between June 2019 and February 2020 (daratumumab available and reimbursed since June 2019).
Results
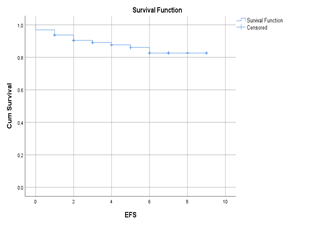
A total of 96 patients with RR myeloma were included. Median age at the start of daratumumab treatment was 66.5 years (range 41 – 86). There were 41 male and 57 female subjects. Median number of previous lines of therapies was 3 (range 2 – 8). 49 patients (51%) previously underwent autologous stem cell transplantation (ASCT) in the first line of treatment (12 patients received tandem ASCT) and 16 patients (17%) as salvage ASCT (6 patients had tandem ASCT). 57 patients (59%) were bortezomib exposed and 31 (32%) lenalidomide exposed. DVd was administered to 36 patients (38%) and DRd to 60 patients (62%). In the DVd group response rate (better or equal to partial response; PR) was 72%, while in the DRd group response rate was 76%. After a median follow up of 6 months, median progression free survival (PFS) was not reached in both groups. During the study, hematologic toxicities were reported for the entire group: anemia, thrombocytopenia and neutropenia in 30, 27 and 26 patients, respectively. Infective complications were reported in 21 patients in total. During the study 12 patients died.
Conclusion
This real-world data analysis confirms that in the setting of RR MM daratumumab has significant efficacy with acceptable and manageable toxicities. Our data support previously reported clinical trials data.
Keyword(s): Monoclonal antibody, Myeloma
Abstract: PB1684
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Multiple myeloma (MM) remains uncurable disease, with multiple relapses and clone evolution leading to refractoriness. However, recently significant therapeutical advancements were achieved leading to improvement of patient’s survival.
Aims
The aim of this analysis was to show real world data on treating multiple myeloma patients with relapsed/refractory (RR) disease with daratumumab.
Methods
We performed a retrospective analysis of outcomes of MM patients treated with daratumumab, in combination with bortezomib and dexamethasone (DVd) or lenalidomide and dexamethasone (DRd) in 13 Croatian hematology centers in the period between June 2019 and February 2020 (daratumumab available and reimbursed since June 2019).
Results
A total of 96 patients with RR myeloma were included. Median age at the start of daratumumab treatment was 66.5 years (range 41 – 86). There were 41 male and 57 female subjects. Median number of previous lines of therapies was 3 (range 2 – 8). 49 patients (51%) previously underwent autologous stem cell transplantation (ASCT) in the first line of treatment (12 patients received tandem ASCT) and 16 patients (17%) as salvage ASCT (6 patients had tandem ASCT). 57 patients (59%) were bortezomib exposed and 31 (32%) lenalidomide exposed. DVd was administered to 36 patients (38%) and DRd to 60 patients (62%). In the DVd group response rate (better or equal to partial response; PR) was 72%, while in the DRd group response rate was 76%. After a median follow up of 6 months, median progression free survival (PFS) was not reached in both groups. During the study, hematologic toxicities were reported for the entire group: anemia, thrombocytopenia and neutropenia in 30, 27 and 26 patients, respectively. Infective complications were reported in 21 patients in total. During the study 12 patients died.

Conclusion
This real-world data analysis confirms that in the setting of RR MM daratumumab has significant efficacy with acceptable and manageable toxicities. Our data support previously reported clinical trials data.
Keyword(s): Monoclonal antibody, Myeloma


