
Contributions
Abstract: PB1676
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Digital applications in healthcare (e-health) are increasingly used. In solid oncology, implementing electronic patient-reported symptom monitoring was associated with improved survival. Currently, medication support applications are not available. Patients with multiple myeloma are faced with complicated treatment regimens. They may receive various treatment lines containing several anti-myeloma and supportive care medications. Treatment is based on tumor and patient characteristics, but also on patient preferences and side effects.
Aims
To explore the needs of patients with Multiple Myeloma and their health care providers, with regard to supportive care using eHealth. Additionally, to design an eHealth application to address these needs and evaluate its usability and feasibility.
Methods
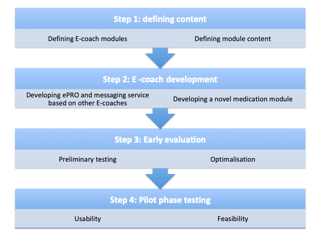
This study was performed in a large non-academic hospital, collaborating with an experienced E-Coach developer. First, to define the current standard of care the Multiple Myeloma treatment pathway was identified within a value based healthcare (VBHC) trajectory. Second, health care providers’ (physicians, nurses, pharmacists and supportive staff) needs were explored during five focus group sessions. Patients and their spouses participated in the third session. The focus group topic agenda was based on the VBHC trajectory outcome and on two surveys amongst 18 patients with Multiple Myeloma. The first survey explored the current experience with care delivery and the needs for the eHealth application. The second evaluated potential patient reported outcomes (PROs), based on the EORTC QLQ-C30, MARS-5 and patient reported experience measures (PREMs). Finally, the application, called the ‘E-Coach’, was developed in a multi step process (figure 1): step 1, defining content; step 2, e-coach development; step 3, early evaluation; and step 4, pilot phase testing.
Results
Two sets of PROs were defined. First, a large set of PROMs and PREMs to perform periodic evaluations at large intervals (e.g.: 3 or 6 months). Second, a short list of PROs and one blank space for personal suggestions to prepare outpatient clinic visits. The participants expressed a need for medication support and overview and for a messaging service. The application was developed, using several modules. The messaging and PRO questionnaire modules were integrated in already existing application modules. Additionally, the medication module was developed during an iterative process with the health care providers. Then, a first version of the Multiple Myeloma E-coach was tested in a pilot study. During eight weeks, the application was used by twenty patients and a clinical multidisciplinary team (clinicians, nurses and pharmacists). Eighty-three percent of participants were satisfied using the E-coach. Fifteen recommendations were done for improvements, such as individualizing the built-in tool to check when medicines had been taken. Some patients preferred to check every medicine they took; others preferred to check several at the same time. The health care providers recommended creating a dashboard, for overview of all PROs and medication adherence. Based on all recommendations, the E-coach was adapted.
Conclusion
Using an approach that involved all relevant stakeholders, we developed a multi-module E-coach for patients with Multiple Myeloma receiving complicated treatment regimens. Most notable, we developed a medication module for support and overview of medication. To further evaluate the E-coach, a randomized controlled trial is planned to compare implementation of the E-coach with regular care (EudraCT 2020-005267-31).
Keyword(s): Multiple myeloma
Abstract: PB1676
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Digital applications in healthcare (e-health) are increasingly used. In solid oncology, implementing electronic patient-reported symptom monitoring was associated with improved survival. Currently, medication support applications are not available. Patients with multiple myeloma are faced with complicated treatment regimens. They may receive various treatment lines containing several anti-myeloma and supportive care medications. Treatment is based on tumor and patient characteristics, but also on patient preferences and side effects.
Aims
To explore the needs of patients with Multiple Myeloma and their health care providers, with regard to supportive care using eHealth. Additionally, to design an eHealth application to address these needs and evaluate its usability and feasibility.
Methods
This study was performed in a large non-academic hospital, collaborating with an experienced E-Coach developer. First, to define the current standard of care the Multiple Myeloma treatment pathway was identified within a value based healthcare (VBHC) trajectory. Second, health care providers’ (physicians, nurses, pharmacists and supportive staff) needs were explored during five focus group sessions. Patients and their spouses participated in the third session. The focus group topic agenda was based on the VBHC trajectory outcome and on two surveys amongst 18 patients with Multiple Myeloma. The first survey explored the current experience with care delivery and the needs for the eHealth application. The second evaluated potential patient reported outcomes (PROs), based on the EORTC QLQ-C30, MARS-5 and patient reported experience measures (PREMs). Finally, the application, called the ‘E-Coach’, was developed in a multi step process (figure 1): step 1, defining content; step 2, e-coach development; step 3, early evaluation; and step 4, pilot phase testing.
Results
Two sets of PROs were defined. First, a large set of PROMs and PREMs to perform periodic evaluations at large intervals (e.g.: 3 or 6 months). Second, a short list of PROs and one blank space for personal suggestions to prepare outpatient clinic visits. The participants expressed a need for medication support and overview and for a messaging service. The application was developed, using several modules. The messaging and PRO questionnaire modules were integrated in already existing application modules. Additionally, the medication module was developed during an iterative process with the health care providers. Then, a first version of the Multiple Myeloma E-coach was tested in a pilot study. During eight weeks, the application was used by twenty patients and a clinical multidisciplinary team (clinicians, nurses and pharmacists). Eighty-three percent of participants were satisfied using the E-coach. Fifteen recommendations were done for improvements, such as individualizing the built-in tool to check when medicines had been taken. Some patients preferred to check every medicine they took; others preferred to check several at the same time. The health care providers recommended creating a dashboard, for overview of all PROs and medication adherence. Based on all recommendations, the E-coach was adapted.

Conclusion
Using an approach that involved all relevant stakeholders, we developed a multi-module E-coach for patients with Multiple Myeloma receiving complicated treatment regimens. Most notable, we developed a medication module for support and overview of medication. To further evaluate the E-coach, a randomized controlled trial is planned to compare implementation of the E-coach with regular care (EudraCT 2020-005267-31).
Keyword(s): Multiple myeloma


