
Contributions
Abstract: PB1654
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Daratumumab, bortezomib and dexamethasone (DVd) is a therapy that has demonstrated significant clinical activity in relapsed/ refractory multiple myeloma (RRMM). In April 2017, DVd was approved by the European Medicines Agency (EMA). In June 2020, subcutaneous (SC) daratumumab gained EMA approval.
Aims
To analyze the clinical characteristics, response rates and treatment emergent toxicity of DVd combined therapy in two European centres.
Methods
This two-centre retrospective study includes 25 RRMM patients who started DVd therapy between July 2018 and November 2020 at Guy’s Hospital (London) or Hospital Principe de Asturias (Madrid). The patients' data were reviewed using electronic records. Response outcomes were assessed for patients who received ≥1 cycles.
Results
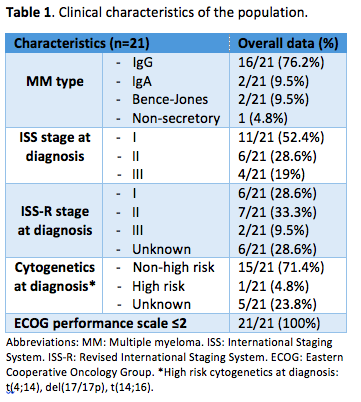
The median age at the start of therapy was 62 (range 42-86), with 15 (60%) male patients. Clinical characteristics are shown in Table 1. Twenty (80%) patients had 1 previous line of therapy: 5 (25%) VMP (bortezomib, melphalan, prednisone), 5 (25%) VTd (bortezomib, thalidomide, dexamethasone), 3 (15%) VCd (bortezomib, cyclophosphamide, dexamethasone), 1 (5%) Vd (bortezomib, dexamethasone), 1 (5%) CRd (carfilzomib, lenalidomide, dexamethasone) and 4 (20%) a clinical trial. Five (20%) patients had 2 prior lines. Fourteen (56%) had undergone autologous stem cell transplant. Overall, 21/25 (84%) patients had been exposed to bortezomib: overall response rate (ORR) was 90.5% and 1 (4.8%) was bortezomib-refractory in 1st line. Median progression free survival (PFS) was 43.1 months (m) and median treatment-free interval was 33.2 m.
All patients had disease progression and 1 (4%) extramedullary disease prior to starting DVd. Median time on treatment was 9.6 m (0.2-29.4), with a median number of cycles of 12 (2-33). Seven (28%) patients discontinued therapy: 5 (71.4%) due to progressive disease, 1 (14.3%) for patient’s choice and 1 (14.3%) died on treatment (due to SARs-CoV2-related pneumonia).
At a median follow-up of 11 m (1.8-29.4), 24 (95.2%) patients have completed ≥1 cycles. ORR is 83.3%, with very good partial response (VGPR) in 10/24 (41.7%) patients; only 2 (8.3%) patients had no response. The patient who was bortezomib-refractory in 1st line has a partial response (PR) so far. Median time to PR in the population is 36 days (15-120). PFS and overall survival (OS) rates are 73.7% and 90% at 11 m.
Daratumumab administration was SC in 6 (24%) patients, and was switched from intravenous (IV) to SC in 8 (32%) patients. Eight (32%) patients had a first-dose infusion related reaction (IRR) with daratumumab, in all cases being IV. In 3 (12%) it had grade 3 (hypertension) and infusion could be resumed.
Common (≥15%) AEs were: lymphopenia (76%), anaemia (64%), thrombocytopenia (60%), neutropenia (20%), peripheral sensory neuropathy (32%) and infections (36%), most of them SARs-CoV2 related (44.4%).
Grade ≥3 AEs occurred in 12 (48%) patients. Three (25%) developed thrombocytopenia, 1 (8.3%) anaemia, 3 (25%) neutropenia and 6 (50%) lymphopenia. Moreover, 3 (25%) patients had SARs-CoV2-related pneumonia (in 1 leading to death), 1 (8.3%) neutropenic sepsis secondary to pneumonia (causing death), 1 (8.3%) sepsis secondary to jaw infection and 1 (8.3%) non-pneumonic respiratory tract infection.
Conclusion
Daratumumab combined with bortezomib and dexamethasone as salvage therapy in MM demonstrates encouraging outcome results in our population, similar to those exposed in the analysis of the phase III CASTOR study. It shows an acceptable safety profile, with no IRRs to the moment since the introduction of SC daratumumab.
Keyword(s): Bortezomib, CD38, Multiple myeloma, Refractory
Abstract: PB1654
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Daratumumab, bortezomib and dexamethasone (DVd) is a therapy that has demonstrated significant clinical activity in relapsed/ refractory multiple myeloma (RRMM). In April 2017, DVd was approved by the European Medicines Agency (EMA). In June 2020, subcutaneous (SC) daratumumab gained EMA approval.
Aims
To analyze the clinical characteristics, response rates and treatment emergent toxicity of DVd combined therapy in two European centres.
Methods
This two-centre retrospective study includes 25 RRMM patients who started DVd therapy between July 2018 and November 2020 at Guy’s Hospital (London) or Hospital Principe de Asturias (Madrid). The patients' data were reviewed using electronic records. Response outcomes were assessed for patients who received ≥1 cycles.
Results
The median age at the start of therapy was 62 (range 42-86), with 15 (60%) male patients. Clinical characteristics are shown in Table 1. Twenty (80%) patients had 1 previous line of therapy: 5 (25%) VMP (bortezomib, melphalan, prednisone), 5 (25%) VTd (bortezomib, thalidomide, dexamethasone), 3 (15%) VCd (bortezomib, cyclophosphamide, dexamethasone), 1 (5%) Vd (bortezomib, dexamethasone), 1 (5%) CRd (carfilzomib, lenalidomide, dexamethasone) and 4 (20%) a clinical trial. Five (20%) patients had 2 prior lines. Fourteen (56%) had undergone autologous stem cell transplant. Overall, 21/25 (84%) patients had been exposed to bortezomib: overall response rate (ORR) was 90.5% and 1 (4.8%) was bortezomib-refractory in 1st line. Median progression free survival (PFS) was 43.1 months (m) and median treatment-free interval was 33.2 m.
All patients had disease progression and 1 (4%) extramedullary disease prior to starting DVd. Median time on treatment was 9.6 m (0.2-29.4), with a median number of cycles of 12 (2-33). Seven (28%) patients discontinued therapy: 5 (71.4%) due to progressive disease, 1 (14.3%) for patient’s choice and 1 (14.3%) died on treatment (due to SARs-CoV2-related pneumonia).
At a median follow-up of 11 m (1.8-29.4), 24 (95.2%) patients have completed ≥1 cycles. ORR is 83.3%, with very good partial response (VGPR) in 10/24 (41.7%) patients; only 2 (8.3%) patients had no response. The patient who was bortezomib-refractory in 1st line has a partial response (PR) so far. Median time to PR in the population is 36 days (15-120). PFS and overall survival (OS) rates are 73.7% and 90% at 11 m.
Daratumumab administration was SC in 6 (24%) patients, and was switched from intravenous (IV) to SC in 8 (32%) patients. Eight (32%) patients had a first-dose infusion related reaction (IRR) with daratumumab, in all cases being IV. In 3 (12%) it had grade 3 (hypertension) and infusion could be resumed.
Common (≥15%) AEs were: lymphopenia (76%), anaemia (64%), thrombocytopenia (60%), neutropenia (20%), peripheral sensory neuropathy (32%) and infections (36%), most of them SARs-CoV2 related (44.4%).
Grade ≥3 AEs occurred in 12 (48%) patients. Three (25%) developed thrombocytopenia, 1 (8.3%) anaemia, 3 (25%) neutropenia and 6 (50%) lymphopenia. Moreover, 3 (25%) patients had SARs-CoV2-related pneumonia (in 1 leading to death), 1 (8.3%) neutropenic sepsis secondary to pneumonia (causing death), 1 (8.3%) sepsis secondary to jaw infection and 1 (8.3%) non-pneumonic respiratory tract infection.

Conclusion
Daratumumab combined with bortezomib and dexamethasone as salvage therapy in MM demonstrates encouraging outcome results in our population, similar to those exposed in the analysis of the phase III CASTOR study. It shows an acceptable safety profile, with no IRRs to the moment since the introduction of SC daratumumab.
Keyword(s): Bortezomib, CD38, Multiple myeloma, Refractory


