
Contributions
Abstract: PB1653
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Results from clinical trials in patients newly diagnosed with multiple myeloma (MM) or those with relapsed/refractory MM (including trials investigating add-on monoclonal antibodies to standard of care regimens), as well as retrospective analyses from clinical practice, have demonstrated a consistent association between minimal residual disease (MRD) negativity in MM and sustained clinical response to therapies. In these studies, depth of clinical response as measured by MRD predicted progression-free survival and overall survival. While these data indicate that MRD status is prognostic in clinical practice and may represent a valuable surrogate efficacy endpoint in clinical trials, to date, results from large-scale randomized clinical trials of MRD-directed therapy are lacking. In the absence of these data, a Delphi survey to establish consensus regarding the clinical practice utility of MRD in MM may serve as a bridge to help close this knowledge gap. Here we describe the interim results of a modified Delphi analysis conducted among representative experts in the field of MM across Europe.
Aims
To establish whether there is consensus on the relevance of determining MRD status in MM clinical practice in assessing and confirming response to therapy, and to assist physicians in implementing MRD assessment in the management of patients with MM.
Methods
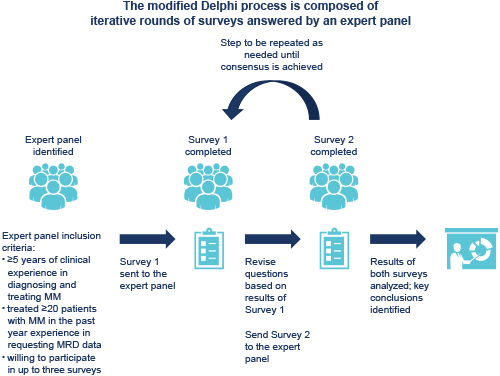
The modified Delphi process is composed of two or more rounds of surveys answered by an expert panel. The expert panel comprises invited, currently practicing, hematological oncologists who fit all inclusion criteria (see Figure). A panel of 11 advisors across 8 European countries developed the questionnaires, identified and invited expert panel members, completed the surveys, and analyzed the results. Questions from Survey 1 were divided into 6 categories: screening and respondent demographics; perceptions regarding the potential use of MRD in clinical practice; actual use of MRD; barriers to routine MRD assessment; guidelines concerning the use of MRD; and educational needs concerning the use of MRD in MM. Advisors defined consensus a priori as 75% agreement or disagreement by the expert panel. Participants’ contact details and survey data were handled in accordance with local regulatory requirements. This analysis focuses on the results from the categories on guidelines and educational needs concerning the use of MRD in MM (as stated in the categories above).
Results
Sixty-one experts (11 advisors; 50 invited) from 16 Western European countries completed Survey 1. Half (50%) reported that MRD was covered in either the prognostic, treatment, or both guidelines that they used. 64% felt suitably informed regarding the use of MRD in MM and were confident using it in a variety of clinical situations, while only 16% felt other clinicians in their country were suitably informed. There was consensus among the experts that clinicians in their country would benefit from education/resources in the use of MRD in informing treatment decisions (92%), and guidelines on the use of MRD in MM (75%).
Conclusion
As the treatment of MM continues to evolve, guidelines on using MRD in the clinic are increasingly necessary. Results from a Delphi survey of expert MM clinicians suggest a lack of MRD practice guidelines, and consensus that clinicians across Europe were not suitably informed regarding the clinical use of MRD in MM. These preliminary results highlight the importance of establishing guidelines and providing education on MRD in MM; final Delphi results will provide areas of expert consensus on MRD utility in clinical practice.
Keyword(s): Minimal residual disease (MRD), Multiple myeloma, Prognostic
Abstract: PB1653
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Results from clinical trials in patients newly diagnosed with multiple myeloma (MM) or those with relapsed/refractory MM (including trials investigating add-on monoclonal antibodies to standard of care regimens), as well as retrospective analyses from clinical practice, have demonstrated a consistent association between minimal residual disease (MRD) negativity in MM and sustained clinical response to therapies. In these studies, depth of clinical response as measured by MRD predicted progression-free survival and overall survival. While these data indicate that MRD status is prognostic in clinical practice and may represent a valuable surrogate efficacy endpoint in clinical trials, to date, results from large-scale randomized clinical trials of MRD-directed therapy are lacking. In the absence of these data, a Delphi survey to establish consensus regarding the clinical practice utility of MRD in MM may serve as a bridge to help close this knowledge gap. Here we describe the interim results of a modified Delphi analysis conducted among representative experts in the field of MM across Europe.
Aims
To establish whether there is consensus on the relevance of determining MRD status in MM clinical practice in assessing and confirming response to therapy, and to assist physicians in implementing MRD assessment in the management of patients with MM.
Methods
The modified Delphi process is composed of two or more rounds of surveys answered by an expert panel. The expert panel comprises invited, currently practicing, hematological oncologists who fit all inclusion criteria (see Figure). A panel of 11 advisors across 8 European countries developed the questionnaires, identified and invited expert panel members, completed the surveys, and analyzed the results. Questions from Survey 1 were divided into 6 categories: screening and respondent demographics; perceptions regarding the potential use of MRD in clinical practice; actual use of MRD; barriers to routine MRD assessment; guidelines concerning the use of MRD; and educational needs concerning the use of MRD in MM. Advisors defined consensus a priori as 75% agreement or disagreement by the expert panel. Participants’ contact details and survey data were handled in accordance with local regulatory requirements. This analysis focuses on the results from the categories on guidelines and educational needs concerning the use of MRD in MM (as stated in the categories above).
Results
Sixty-one experts (11 advisors; 50 invited) from 16 Western European countries completed Survey 1. Half (50%) reported that MRD was covered in either the prognostic, treatment, or both guidelines that they used. 64% felt suitably informed regarding the use of MRD in MM and were confident using it in a variety of clinical situations, while only 16% felt other clinicians in their country were suitably informed. There was consensus among the experts that clinicians in their country would benefit from education/resources in the use of MRD in informing treatment decisions (92%), and guidelines on the use of MRD in MM (75%).

Conclusion
As the treatment of MM continues to evolve, guidelines on using MRD in the clinic are increasingly necessary. Results from a Delphi survey of expert MM clinicians suggest a lack of MRD practice guidelines, and consensus that clinicians across Europe were not suitably informed regarding the clinical use of MRD in MM. These preliminary results highlight the importance of establishing guidelines and providing education on MRD in MM; final Delphi results will provide areas of expert consensus on MRD utility in clinical practice.
Keyword(s): Minimal residual disease (MRD), Multiple myeloma, Prognostic


