
Contributions
Abstract: PB1652
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Carfilzomib (K) was approved in combination with dexamethasone (d), i.e. Kd, or with lenalidomide (R) and d, i.e. KRd, in Europe during a large, prospective, observational study (NCT03091127) that included 701 patients (pts) with relapsed or refractory multiple myeloma (RRMM) across 10 European countries and Israel.
However, in participating Central Eastern European (CEE) countries and Israel, usage of K-based regimens was restricted in routine practice: in Bulgaria and Romania, Kd was reimbursed for use in second line of therapy (LoT) and KRd access was limited by R availability for private purchase; in Czech Republic (CZ), only KRd was reimbursed. In Israel, KRd was reimbursed in second LoT, and Kd in third LoT.
Aims
To understand effectiveness, safety and usage of K-based regimens according to routine practice in selected CEE countries and Israel during study NCT03091127.
Methods
Adults with ≥1 prior LoT (pLoT) and ≥1 K dose received in routine care were eligible. Data were collected and analysed as previously described (Terpos et al., Blood 2020. 136 [Suppl_1]: 38-39).
Results
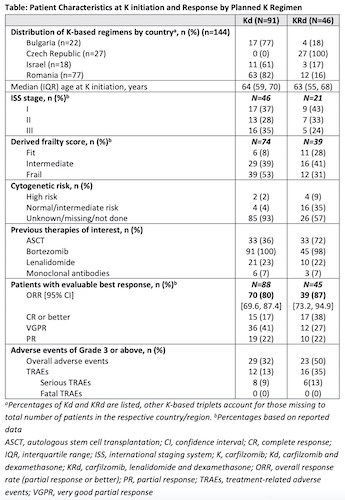
Of the 144 pts enrolled in CEE and Israel, 63% received Kd and 32% KRd. The remaining 5% received other K-based triplets (Kd+cyclophosphamide, Kd+thalidomide) not further described here. Pts characteristics and results are summarized in Table. Across countries, Kd was mainly used, except for CZ (KRd only). Median pts age was comparable in Kd and KRd cohorts (64 vs 63 years).
Kd pts had received a median of 2 pLoTs, 36% had a prior autologous stem cell transplantation (ASCT). Nearly all Kd pts were exposed to bortezomib (V) in a pLoT and over half (53%) of them were V-refractory. Over one-fifth (23%) were exposed to R and 81% of these were R-refractory.
KRd pts had received a median of 1 pLoT and 72% had a prior ASCT. All KRd pts had a prior V-exposure with one-third being V-refractory. Prior R-exposure was reported in 22% of pts, of whom 40% were R-refractory. In this population, overall response rates (ORRs) were high regardless of K-based regimen (Kd: 80%; KRd: 87%).
The mean K dose intensity received relative to EU label for Kd (20/56 mg/m2 twice weekly [Q2W] for K) and KRd (20/27 mg/m2Q2W for K) was 81% and 83%, respectively. The Kaplan-Meier median estimate of treatment duration was 9.7 months (95% CI: 7.2, 12.0) for Kd and 13.6 months (95% CI: 8.8, 16.6) for KRd.
Treatment-related adverse events (TRAEs) of grade 3 or above (Gr3+) were reported in 13% of Kd and 35% of KRd pts, including 9% and 13% of serious TRAEs, respectively. The most frequent Gr3+ TRAEs were blood cytopenias (2% Kd, 24% KRd), infections (3% Kd, 11% KRd), respiratory and vascular disorders (2% Kd, 4% KRd). Of the fatal events (8 Kd and 1 KRd), none were considered treatment related.
Conclusion
In CEE and Israel, usage of K-based regimens (32% KRd, 63% Kd) differed from that in the overall study population (55% KRd, 39% Kd) probably due to country-specific reimbursement regulations at time of study. Compared with the overall study population, pts enrolled in CEE and Israel were generally younger (Kd: 64 vs 68; KRd: 63 vs 65) and Kd was used in earlier lines (median number of pLoTs: 2 vs 3). Irrespective of K-based regimens, high response rates (over 80%) and deep responses (≥VGPR: Kd 58%, KRd 64%) were achieved over long treatment durations. K-based regimens were well tolerated (TRAE G3+: 13% Kd, 35% KRd).
Keyword(s): Clinical data, Multiple myeloma, Proteasome inhibitor
Abstract: PB1652
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Carfilzomib (K) was approved in combination with dexamethasone (d), i.e. Kd, or with lenalidomide (R) and d, i.e. KRd, in Europe during a large, prospective, observational study (NCT03091127) that included 701 patients (pts) with relapsed or refractory multiple myeloma (RRMM) across 10 European countries and Israel.
However, in participating Central Eastern European (CEE) countries and Israel, usage of K-based regimens was restricted in routine practice: in Bulgaria and Romania, Kd was reimbursed for use in second line of therapy (LoT) and KRd access was limited by R availability for private purchase; in Czech Republic (CZ), only KRd was reimbursed. In Israel, KRd was reimbursed in second LoT, and Kd in third LoT.
Aims
To understand effectiveness, safety and usage of K-based regimens according to routine practice in selected CEE countries and Israel during study NCT03091127.
Methods
Adults with ≥1 prior LoT (pLoT) and ≥1 K dose received in routine care were eligible. Data were collected and analysed as previously described (Terpos et al., Blood 2020. 136 [Suppl_1]: 38-39).
Results
Of the 144 pts enrolled in CEE and Israel, 63% received Kd and 32% KRd. The remaining 5% received other K-based triplets (Kd+cyclophosphamide, Kd+thalidomide) not further described here. Pts characteristics and results are summarized in Table. Across countries, Kd was mainly used, except for CZ (KRd only). Median pts age was comparable in Kd and KRd cohorts (64 vs 63 years).
Kd pts had received a median of 2 pLoTs, 36% had a prior autologous stem cell transplantation (ASCT). Nearly all Kd pts were exposed to bortezomib (V) in a pLoT and over half (53%) of them were V-refractory. Over one-fifth (23%) were exposed to R and 81% of these were R-refractory.
KRd pts had received a median of 1 pLoT and 72% had a prior ASCT. All KRd pts had a prior V-exposure with one-third being V-refractory. Prior R-exposure was reported in 22% of pts, of whom 40% were R-refractory. In this population, overall response rates (ORRs) were high regardless of K-based regimen (Kd: 80%; KRd: 87%).
The mean K dose intensity received relative to EU label for Kd (20/56 mg/m2 twice weekly [Q2W] for K) and KRd (20/27 mg/m2Q2W for K) was 81% and 83%, respectively. The Kaplan-Meier median estimate of treatment duration was 9.7 months (95% CI: 7.2, 12.0) for Kd and 13.6 months (95% CI: 8.8, 16.6) for KRd.
Treatment-related adverse events (TRAEs) of grade 3 or above (Gr3+) were reported in 13% of Kd and 35% of KRd pts, including 9% and 13% of serious TRAEs, respectively. The most frequent Gr3+ TRAEs were blood cytopenias (2% Kd, 24% KRd), infections (3% Kd, 11% KRd), respiratory and vascular disorders (2% Kd, 4% KRd). Of the fatal events (8 Kd and 1 KRd), none were considered treatment related.

Conclusion
In CEE and Israel, usage of K-based regimens (32% KRd, 63% Kd) differed from that in the overall study population (55% KRd, 39% Kd) probably due to country-specific reimbursement regulations at time of study. Compared with the overall study population, pts enrolled in CEE and Israel were generally younger (Kd: 64 vs 68; KRd: 63 vs 65) and Kd was used in earlier lines (median number of pLoTs: 2 vs 3). Irrespective of K-based regimens, high response rates (over 80%) and deep responses (≥VGPR: Kd 58%, KRd 64%) were achieved over long treatment durations. K-based regimens were well tolerated (TRAE G3+: 13% Kd, 35% KRd).
Keyword(s): Clinical data, Multiple myeloma, Proteasome inhibitor


