
Contributions
Abstract: PB1648
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Venous thromboembolism (VTE) is a significant complication for patients with multiple myeloma (MM) receiving immunomodulatory drugs (IMiDs). The International Myeloma Working Group (IMWG) developed guidelines recommending primary thromboprophylaxis in those identified to be at high risk of VTE according to the presence of risk factors. However, these guidelines lack validation and do not take ethnic differences into account. There is no validated clinical model predicting VTE in this population in China.
Aims
This study aimed to assess the frequency of VTE and associated risk factors and derive a new risk assessment model for IMiD-associated VTE in Chinese MM patients.
Methods
Patients with MM receiving IMiDs were selected from 16 centres in China between 2010 and 2020. We assessed the incidence of VTE and the associated clinical parameters. The primary endpoint was the rate of symptomatic VTE. Secondary endpoints were associations between VTE and clinical factors, including age, sex, disease characteristics and duration, history of VTE, immobilization, comorbidities, treatment regimens, and laboratory parameters.
A multivariable cause-specific Cox regression model was used for model development.
Results
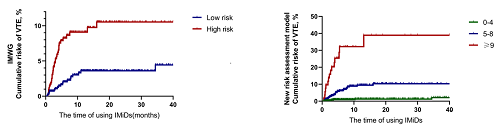
A total of 1137 myeloma patients were followed up for a median of 8.51 months (range 0.46-180.01 months), and 52 (4.6%) developed VTE, which was lower than the rate found in Western countries. In addition, 41 patients developed VTE within 6 months of treatment with IMiDs. The sites for VTE included the lungs (6%), lower extremities (71%), upper extremities (11%), and central venous catheter (12%). Thromboprophylaxis with aspirin or warfarin did not reduce the risk of VTE; the rates of VTE with or without aspirin or warfarin treatment were 5.2% and 4.1% (p=0.377), respectively. Multivariate analysis determined that an ECOG score≥2, history of VTE, D-dimer>2.42 mg/L, use of IMiDs during induction therapy, diabetes and the use of lenalidomide, dexamethasone, anthracyclines or erythropoietin were significant risk factors for VTE. We derived a new risk assessment model that included these 9 clinical variables. The model stratified approximately 32.1% of patients as high-risk. In contrast, the IMWG stratification method only classified 9.1% of patients as high-risk. A high-risk group for VTE among MM patients receiving IMiDs was identified, and a larger study is needed to confirm these findings.
Conclusion
The new risk assessment model score outperformed the current IMWG guidelines in the risk stratification of patients with MM receiving IMiD therapy in China. A high-risk group for VTE among MM patients receiving IMiDs was identified, and a larger study is needed to confirm these findings.
Keyword(s): Myeloma, Venous thromboembolism
Abstract: PB1648
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Venous thromboembolism (VTE) is a significant complication for patients with multiple myeloma (MM) receiving immunomodulatory drugs (IMiDs). The International Myeloma Working Group (IMWG) developed guidelines recommending primary thromboprophylaxis in those identified to be at high risk of VTE according to the presence of risk factors. However, these guidelines lack validation and do not take ethnic differences into account. There is no validated clinical model predicting VTE in this population in China.
Aims
This study aimed to assess the frequency of VTE and associated risk factors and derive a new risk assessment model for IMiD-associated VTE in Chinese MM patients.
Methods
Patients with MM receiving IMiDs were selected from 16 centres in China between 2010 and 2020. We assessed the incidence of VTE and the associated clinical parameters. The primary endpoint was the rate of symptomatic VTE. Secondary endpoints were associations between VTE and clinical factors, including age, sex, disease characteristics and duration, history of VTE, immobilization, comorbidities, treatment regimens, and laboratory parameters.
A multivariable cause-specific Cox regression model was used for model development.
Results
A total of 1137 myeloma patients were followed up for a median of 8.51 months (range 0.46-180.01 months), and 52 (4.6%) developed VTE, which was lower than the rate found in Western countries. In addition, 41 patients developed VTE within 6 months of treatment with IMiDs. The sites for VTE included the lungs (6%), lower extremities (71%), upper extremities (11%), and central venous catheter (12%). Thromboprophylaxis with aspirin or warfarin did not reduce the risk of VTE; the rates of VTE with or without aspirin or warfarin treatment were 5.2% and 4.1% (p=0.377), respectively. Multivariate analysis determined that an ECOG score≥2, history of VTE, D-dimer>2.42 mg/L, use of IMiDs during induction therapy, diabetes and the use of lenalidomide, dexamethasone, anthracyclines or erythropoietin were significant risk factors for VTE. We derived a new risk assessment model that included these 9 clinical variables. The model stratified approximately 32.1% of patients as high-risk. In contrast, the IMWG stratification method only classified 9.1% of patients as high-risk. A high-risk group for VTE among MM patients receiving IMiDs was identified, and a larger study is needed to confirm these findings.

Conclusion
The new risk assessment model score outperformed the current IMWG guidelines in the risk stratification of patients with MM receiving IMiD therapy in China. A high-risk group for VTE among MM patients receiving IMiDs was identified, and a larger study is needed to confirm these findings.
Keyword(s): Myeloma, Venous thromboembolism


