
Contributions
Abstract: PB1646
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Bortezomib, as an intravenous proteasome inhibitor (PI) is important in the treatment of multiple myeloma (MM), but it always discontinued by adverse event (AE).
Aims
Assessing in-class transition (iCT) from intravenous bortezomib-based induction to all-oral ixazomib-based regimen is a good method for improving PI-based continuous therapy with efficiency, safety and convenience.
Methods
In this real-world retrospective study from Chinese seven hospitals during Oct, 2017-Jan, 2021, we analysed newly diagnosed multiple myeloma (NDMM) and first relapsed multiple myeloma (FRMM)patients without transplantation, who achieved at least partial response of bortezomib-based introduction and then received ixazomib-based regimen for 2 years or until progression/intolerant toxicity. Primary endpoint: progression-free survival (PFS). Key second endpoints included response rate, therapy duration and AE.
Results
183 patients were enrolled in this study (NDMM 76%, FRMM 24%, median age 63 years; 42% ≥ 65 years; 10% ≥ 75 years; 51% male; IgG/IgA/IgD/light chain /non-secretory/others 50%/22%/2%/22%/1%/3%; ISS stage I/II/III 16%/37%/47%; R-ISS stage was estimated in 107 patients, stage I/II/III 9%/54%/37%). 48% high risk (HR,59/123) and 52% standard risk (SR,64/123) were tested by metaphase fluorescence in situ hybridization (M-FISH) according to Mayo clinic risk stratification.
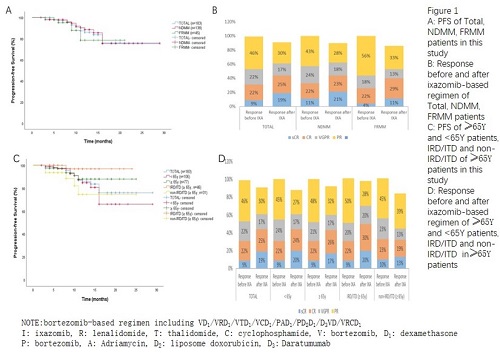
183 patients received median 4 cycles (1-14) of bortezomib-based regimen, and median 4 cycles (1-20) of iCT regimen including I/ ID1/IRD1/ITD1/ICD1. Median duration of total PI therapy was 10 months and of iCT regimen was 4 months. With 13 months median follow-up, 18 month PFS rate was 80% (95% confidence interval,67-93) from the start of bortezomib-based induction and 78% (95% confidence interval,70-94) from the start of iCT (Figure 1A), 66% patients remain on therapy at data cutoff. There’s no significance in PFS between NDMM and FRMM, between HR and SR MM patients (P=0.419, P=0.105, Figure 1A, Figure 1C). Before iCT, ORR rate was 100% (sCR 9%; CR 22%; VGPR 22%; PR 47%) and 90% (sCR 19%; CR 25%; VGPR 19%; PR 27%) after iCT. There were 50 PR improved to more than VGPR (Figure 1B). ORR of HR and SR patients were 100% and 83%, 100% and 94%, before and after iCT, respectively (Figure 1D).
Among 113 patients who transited to IRD1/ITD1 regimen, medium duration of total PI therapy was 9 months and of iCT regimen was 3 months, 77% of patients remain on therapy (Figure 1C). ORR rate was 100% and 92% before and after iCT, respectively (Figure 1D). ORR of HR (23/52), of SR patients (29/52) patients were 100% and 96%, 100% and 86% before and after iCT, respectively. For those non-transplantation-eligible (NTE, ≥65 years old) patients, medium duration of total PI therapy was 9 months and of iCT regimen was 4 months (Figure 1E). PFS and ORR rate of IRD1/ITD1 group are better than non-IRD1/ITD1 group (P=0.023, Figure 1E; ORR rate still 98% after iCT, Figure 1F).
The ixazomib-safety profile was consistent with previous clinical trial data. 54% of patients had any grade AEs. In which, the most frequent grade <=2 AEs were peripheral neuropathy (PN) (37%, 50 patients began before iCT), nausea and vomiting (16%), diarrhea (13%). Grade 3/4 AEs were agranulocytosis (1%), rash (1%), pneumonia (1%), PN (1%). The most often treatment discontinued AEs is PN.
Conclusion
In real-world China MM population, NDMM and FRMM patients are sensitive to PI-based continuous therapy with satisfied response rate; iCT can permit prolonged PI-based therapy with promising efficacy and tolerated adverse events.
Keyword(s): Multiple myeloma, Proteasome inhibitor
Abstract: PB1646
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Bortezomib, as an intravenous proteasome inhibitor (PI) is important in the treatment of multiple myeloma (MM), but it always discontinued by adverse event (AE).
Aims
Assessing in-class transition (iCT) from intravenous bortezomib-based induction to all-oral ixazomib-based regimen is a good method for improving PI-based continuous therapy with efficiency, safety and convenience.
Methods
In this real-world retrospective study from Chinese seven hospitals during Oct, 2017-Jan, 2021, we analysed newly diagnosed multiple myeloma (NDMM) and first relapsed multiple myeloma (FRMM)patients without transplantation, who achieved at least partial response of bortezomib-based introduction and then received ixazomib-based regimen for 2 years or until progression/intolerant toxicity. Primary endpoint: progression-free survival (PFS). Key second endpoints included response rate, therapy duration and AE.
Results
183 patients were enrolled in this study (NDMM 76%, FRMM 24%, median age 63 years; 42% ≥ 65 years; 10% ≥ 75 years; 51% male; IgG/IgA/IgD/light chain /non-secretory/others 50%/22%/2%/22%/1%/3%; ISS stage I/II/III 16%/37%/47%; R-ISS stage was estimated in 107 patients, stage I/II/III 9%/54%/37%). 48% high risk (HR,59/123) and 52% standard risk (SR,64/123) were tested by metaphase fluorescence in situ hybridization (M-FISH) according to Mayo clinic risk stratification.
183 patients received median 4 cycles (1-14) of bortezomib-based regimen, and median 4 cycles (1-20) of iCT regimen including I/ ID1/IRD1/ITD1/ICD1. Median duration of total PI therapy was 10 months and of iCT regimen was 4 months. With 13 months median follow-up, 18 month PFS rate was 80% (95% confidence interval,67-93) from the start of bortezomib-based induction and 78% (95% confidence interval,70-94) from the start of iCT (Figure 1A), 66% patients remain on therapy at data cutoff. There’s no significance in PFS between NDMM and FRMM, between HR and SR MM patients (P=0.419, P=0.105, Figure 1A, Figure 1C). Before iCT, ORR rate was 100% (sCR 9%; CR 22%; VGPR 22%; PR 47%) and 90% (sCR 19%; CR 25%; VGPR 19%; PR 27%) after iCT. There were 50 PR improved to more than VGPR (Figure 1B). ORR of HR and SR patients were 100% and 83%, 100% and 94%, before and after iCT, respectively (Figure 1D).
Among 113 patients who transited to IRD1/ITD1 regimen, medium duration of total PI therapy was 9 months and of iCT regimen was 3 months, 77% of patients remain on therapy (Figure 1C). ORR rate was 100% and 92% before and after iCT, respectively (Figure 1D). ORR of HR (23/52), of SR patients (29/52) patients were 100% and 96%, 100% and 86% before and after iCT, respectively. For those non-transplantation-eligible (NTE, ≥65 years old) patients, medium duration of total PI therapy was 9 months and of iCT regimen was 4 months (Figure 1E). PFS and ORR rate of IRD1/ITD1 group are better than non-IRD1/ITD1 group (P=0.023, Figure 1E; ORR rate still 98% after iCT, Figure 1F).
The ixazomib-safety profile was consistent with previous clinical trial data. 54% of patients had any grade AEs. In which, the most frequent grade <=2 AEs were peripheral neuropathy (PN) (37%, 50 patients began before iCT), nausea and vomiting (16%), diarrhea (13%). Grade 3/4 AEs were agranulocytosis (1%), rash (1%), pneumonia (1%), PN (1%). The most often treatment discontinued AEs is PN.

Conclusion
In real-world China MM population, NDMM and FRMM patients are sensitive to PI-based continuous therapy with satisfied response rate; iCT can permit prolonged PI-based therapy with promising efficacy and tolerated adverse events.
Keyword(s): Multiple myeloma, Proteasome inhibitor


