
Contributions
Abstract: PB1643
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Multiple myeloma (MM) remains an incurable disease with significant morbidity and mortality. Daratumumab (Dara)-containing regimens, both in monotherapy and in combinations with other agents, have significantly altered the therapeutic landscape of MM especially in the relapsed setting.
Aims
The aim of this study was to evaluate the outcomes of Dara-containing regimens in the Canadian real-world setting among MM patients, many of whom may have been ineligible for the landmark trials.
Methods
We performed a retrospective study using the Canadian Myeloma Research Group Database (CMRG-DB), containing real-world data on myeloma outcomes in Canadian academic centers. We identified 798 patients previously exposed to Dara up to 31/08/2020, of which 710 were treated in the relapsed setting. This includes patients for whom Dara was added within 6 months from initiation of a treatment and considered the same line of therapy. Objectives were to evaluate progression-free survival (PFS) and overall survival (OS) using 6 different Dara-based regimens (in monotherapy, with cyclophosphamide, with bortezomib, with lenalidomide, with pomalidomide ± cyclophosphamide), considering median prior lines of therapy.
Results
Of the 710 MM patients receiving Dara-based therapy in ³ 2nd-line treatment, 58.5% were male, 18% had high-risk [del17p, t(4;14) and/or t(14;16)] cytogenetic disease and 34% were ISS 3. Overall, the median number of prior lines of therapy was 3 (range 2-11). After a median follow-up of 13 months, the median PFS and median OS for the entire cohort were 13 months (95% CI 11-15) and 38 months (95% CI 31-72), with 23 months and not reached (NR) in 2nd-line, 14 and 38 months in 3rd-line, and 8 and 20 months in 4th-line treatment, respectively.
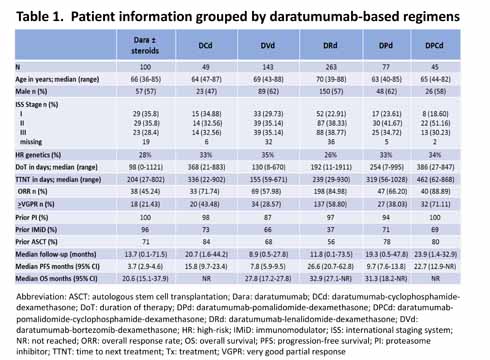
Additional demographic and outcome data stratified by different Dara-containing regimens are shown in Table 1. Dara in monotherapy ± corticosteroids after a median of 5 prior lines of therapy resulted in a median PFS of 3.7 months and median OS of 20.6 months, similar to the SIRIUS trial (Lonial et al. Lancet 2016). The inclusion of cyclophosphamide with Dara after a median of 3 prior lines of therapy resulted in a median PFS of 15.8 months (median OS NR). DVd, after a median of 3 prior lines of therapy, resulted in a median PFS of 7.8 months and median OS of 27.8 months -- significantly less than the results in the latest CASTOR trial update (Mateos et al. Clin Lymphoma Myeloma Leuk 2020) although their patients were less pre-treated. DRd, after a median of 2 prior lines of therapy, resulted in a median PFS of 26.6 months and median OS of 32.9 months which is significantly inferior to results of the last update of the POLLUX trial (Bahlis et al. Leukemia 2020) although their patients were also less pre-treated. DPd, used after a median of 3 prior lines of therapy, resulted in a median PFS of 9.7 months and median OS of 31.3 months similar to what is expected from the EQUULEUS (Chari et al. Blood 2017) and APOLLO trials (Dimopoulos et al. ASH 2020) after a median of 4 and 2 prior lines of therapy, respectively. The addition of cyclophosphamide to Dara and pomalidomide significantly increased the median PFS (22.7 months) and OS (median NR).
Conclusion
Our results demonstrate that Dara-based therapy is effective in relapsed MM patients even in real-world practice. Our observations suggest that the addition of oral cyclophosphamide to Dara-based therapy results in more favourable outcomes and deserves further evaluation.
Keyword(s): Monoclonal antibody, Multiple myeloma, Relapse, Treatment
Abstract: PB1643
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Multiple myeloma (MM) remains an incurable disease with significant morbidity and mortality. Daratumumab (Dara)-containing regimens, both in monotherapy and in combinations with other agents, have significantly altered the therapeutic landscape of MM especially in the relapsed setting.
Aims
The aim of this study was to evaluate the outcomes of Dara-containing regimens in the Canadian real-world setting among MM patients, many of whom may have been ineligible for the landmark trials.
Methods
We performed a retrospective study using the Canadian Myeloma Research Group Database (CMRG-DB), containing real-world data on myeloma outcomes in Canadian academic centers. We identified 798 patients previously exposed to Dara up to 31/08/2020, of which 710 were treated in the relapsed setting. This includes patients for whom Dara was added within 6 months from initiation of a treatment and considered the same line of therapy. Objectives were to evaluate progression-free survival (PFS) and overall survival (OS) using 6 different Dara-based regimens (in monotherapy, with cyclophosphamide, with bortezomib, with lenalidomide, with pomalidomide ± cyclophosphamide), considering median prior lines of therapy.
Results
Of the 710 MM patients receiving Dara-based therapy in ³ 2nd-line treatment, 58.5% were male, 18% had high-risk [del17p, t(4;14) and/or t(14;16)] cytogenetic disease and 34% were ISS 3. Overall, the median number of prior lines of therapy was 3 (range 2-11). After a median follow-up of 13 months, the median PFS and median OS for the entire cohort were 13 months (95% CI 11-15) and 38 months (95% CI 31-72), with 23 months and not reached (NR) in 2nd-line, 14 and 38 months in 3rd-line, and 8 and 20 months in 4th-line treatment, respectively.
Additional demographic and outcome data stratified by different Dara-containing regimens are shown in Table 1. Dara in monotherapy ± corticosteroids after a median of 5 prior lines of therapy resulted in a median PFS of 3.7 months and median OS of 20.6 months, similar to the SIRIUS trial (Lonial et al. Lancet 2016). The inclusion of cyclophosphamide with Dara after a median of 3 prior lines of therapy resulted in a median PFS of 15.8 months (median OS NR). DVd, after a median of 3 prior lines of therapy, resulted in a median PFS of 7.8 months and median OS of 27.8 months -- significantly less than the results in the latest CASTOR trial update (Mateos et al. Clin Lymphoma Myeloma Leuk 2020) although their patients were less pre-treated. DRd, after a median of 2 prior lines of therapy, resulted in a median PFS of 26.6 months and median OS of 32.9 months which is significantly inferior to results of the last update of the POLLUX trial (Bahlis et al. Leukemia 2020) although their patients were also less pre-treated. DPd, used after a median of 3 prior lines of therapy, resulted in a median PFS of 9.7 months and median OS of 31.3 months similar to what is expected from the EQUULEUS (Chari et al. Blood 2017) and APOLLO trials (Dimopoulos et al. ASH 2020) after a median of 4 and 2 prior lines of therapy, respectively. The addition of cyclophosphamide to Dara and pomalidomide significantly increased the median PFS (22.7 months) and OS (median NR).

Conclusion
Our results demonstrate that Dara-based therapy is effective in relapsed MM patients even in real-world practice. Our observations suggest that the addition of oral cyclophosphamide to Dara-based therapy results in more favourable outcomes and deserves further evaluation.
Keyword(s): Monoclonal antibody, Multiple myeloma, Relapse, Treatment


