Contributions
Abstract: PB1642
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Chimeric antigen receptor T cell (CART) therapy is a groundbreaking approach in the treatment of relapsed/refractory hematological malignancies. Given that multiple myeloma (MM) remains an incurable disease to current standard of care therapies, several investigational CAR-T constructs are in development in clinical trials. However, some MM patients may evolve to light-chain (AL) amyloidosis which is currently an exclusion criterion for these trials.
Aims
We present the first case reported of a patient with systemic AL amyloidosis, evolved from a MM, and successfully treated with CART therapy.
Methods
Our institution has developed an academic second generation humanized 41BB-based CART targeting B cell maturation antigen (BCMA), called ARI-0002h, which is currently being tested on the CARTBCMA-HCP-01 clinical trial for patients with relapsed/refractory MM. The patient underwent treatment with ARI-0002h as a compassionate use after approval by the local Health Care Ethics Committee and the Spanish Drugs Agency. An informed consent form was obtained. Lymphodepletion was performed with fludarabine (90 mg/m2) and cyclophosphamide (900 mg/m2), followed by infusion of 3 x106 ARI-0002h cells/kg in a fractionated manner (3 doses). Patient medical records were collected for analysis.
Results
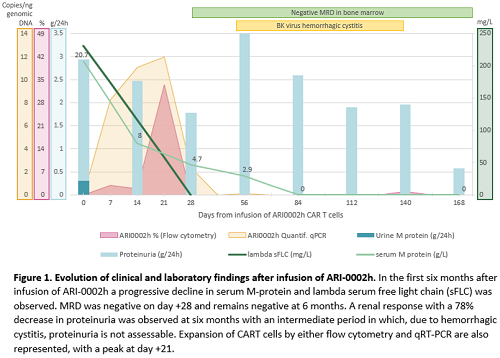
A 61-year-old woman was diagnosed with an IgA-lambda symptomatic MM in 2014. She received several lines of treatment including an autologous stem cell transplantation, proteasome inhibitors, immunomodulatory drugs and the CD38-targeted antibody daratumumab. Despite the previous therapies, the patient relapsed with a serum M-protein of 20.7 g/L, kappa/lambda serum-free light-chain of 2/231 mg/L and bone marrow infiltration by 23% plasma cells (normal FISH), without evidence of extramedullary disease by PET-CT or other CRAB signs. However, she developed edema and significant non-selective albuminuria (24-hour proteinuria of 2626 mg with urinary M-protein of 307 mg). Therefore, subcutaneous fat aspirate and renal biopsy were performed showing amyloid deposits of lambda type. Diagnosis of systemic AL amyloidosis with renal involvement (revised Mayo Stage II) was stablished. Cardiac involvement was ruled out. BCMA expression in bone marrow plasma cells before CART infusion was 23%. The patient received ARI-0002h after lymphodepletion, developing a grade I cytokine release syndrome, treatment-related grade 4 neutropenia and grade 2 thrombocytopenia, with no evidence of neurotoxicity. On day +28 the patient had achieved a hematological complete response (CR) with negative minimal residual disease (MRD) in the bone marrow by next generation flow cytometry. Six months later, the patient remained in MRD negative CR and had achieved a renal response, with a 78% decrease of proteinuria (Figure 1). Renal response at 3 months was not assessable due to a BK virus hemorrhagic cystitis. The highest peak of ARI-0002h expansion was detected at +21 days after infusion and was detectable until day +107 by either flow cytometry and quantitative RT-PCR techniques (Figure 1).
Conclusion
We report the first case to our knowledge of a patient diagnosed with light-chain amyloidosis successfully treated with BCMA-CART therapy. Despite the fact that only a low proportion of clonal plasma cells expressed BCMA, the patient achieved a hematologic CR on day 28 and a renal response at 6 months after CART infusion. Considering the importance of obtaining fast and deep hematologic responses in this disease, CART cells could be a promising therapeutic strategy in AL amyloidosis.
Keyword(s): Amyloidosis, B-cell maturation antigen, CAR-T
Abstract: PB1642
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Chimeric antigen receptor T cell (CART) therapy is a groundbreaking approach in the treatment of relapsed/refractory hematological malignancies. Given that multiple myeloma (MM) remains an incurable disease to current standard of care therapies, several investigational CAR-T constructs are in development in clinical trials. However, some MM patients may evolve to light-chain (AL) amyloidosis which is currently an exclusion criterion for these trials.
Aims
We present the first case reported of a patient with systemic AL amyloidosis, evolved from a MM, and successfully treated with CART therapy.
Methods
Our institution has developed an academic second generation humanized 41BB-based CART targeting B cell maturation antigen (BCMA), called ARI-0002h, which is currently being tested on the CARTBCMA-HCP-01 clinical trial for patients with relapsed/refractory MM. The patient underwent treatment with ARI-0002h as a compassionate use after approval by the local Health Care Ethics Committee and the Spanish Drugs Agency. An informed consent form was obtained. Lymphodepletion was performed with fludarabine (90 mg/m2) and cyclophosphamide (900 mg/m2), followed by infusion of 3 x106 ARI-0002h cells/kg in a fractionated manner (3 doses). Patient medical records were collected for analysis.
Results
A 61-year-old woman was diagnosed with an IgA-lambda symptomatic MM in 2014. She received several lines of treatment including an autologous stem cell transplantation, proteasome inhibitors, immunomodulatory drugs and the CD38-targeted antibody daratumumab. Despite the previous therapies, the patient relapsed with a serum M-protein of 20.7 g/L, kappa/lambda serum-free light-chain of 2/231 mg/L and bone marrow infiltration by 23% plasma cells (normal FISH), without evidence of extramedullary disease by PET-CT or other CRAB signs. However, she developed edema and significant non-selective albuminuria (24-hour proteinuria of 2626 mg with urinary M-protein of 307 mg). Therefore, subcutaneous fat aspirate and renal biopsy were performed showing amyloid deposits of lambda type. Diagnosis of systemic AL amyloidosis with renal involvement (revised Mayo Stage II) was stablished. Cardiac involvement was ruled out. BCMA expression in bone marrow plasma cells before CART infusion was 23%. The patient received ARI-0002h after lymphodepletion, developing a grade I cytokine release syndrome, treatment-related grade 4 neutropenia and grade 2 thrombocytopenia, with no evidence of neurotoxicity. On day +28 the patient had achieved a hematological complete response (CR) with negative minimal residual disease (MRD) in the bone marrow by next generation flow cytometry. Six months later, the patient remained in MRD negative CR and had achieved a renal response, with a 78% decrease of proteinuria (Figure 1). Renal response at 3 months was not assessable due to a BK virus hemorrhagic cystitis. The highest peak of ARI-0002h expansion was detected at +21 days after infusion and was detectable until day +107 by either flow cytometry and quantitative RT-PCR techniques (Figure 1).
Conclusion
We report the first case to our knowledge of a patient diagnosed with light-chain amyloidosis successfully treated with BCMA-CART therapy. Despite the fact that only a low proportion of clonal plasma cells expressed BCMA, the patient achieved a hematologic CR on day 28 and a renal response at 6 months after CART infusion. Considering the importance of obtaining fast and deep hematologic responses in this disease, CART cells could be a promising therapeutic strategy in AL amyloidosis.
Keyword(s): Amyloidosis, B-cell maturation antigen, CAR-T