
Contributions
Abstract: PB1641
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Venetoclax, a selective bcl-2 inhibitor first approved for CLL has been investigated for the treatment of relapsed myeloma patients, and although it failed to show benefit for myeloma patients as a whole, t(11;14) patients demonstrated exceptional responses in the Bellini trial (Lancet Oncol 2020; 21:1630), thus paving the way towards the first genetically targeted treatment in myeloma.
Aims
Off label use of venetoclax is on the rise, even though clinicians have to face unanswered questions regarding the right dosage and length of therapy, as well as the potential for adverse events (AEs), especially infections. Real world data could help to elucidate its optimal use, but is very limited as of yet.
Methods
We addressed all Hungarian centers treating myeloma to evaluate the efficacy and safety of venetoclax in their practice treating t(11;14) myeloma, collecting data about the treatment duration, AEs, dose modifications and treatment discontinuations, and analyzed response rates as well as progression free and overall survivals (PFS, OS).
Results
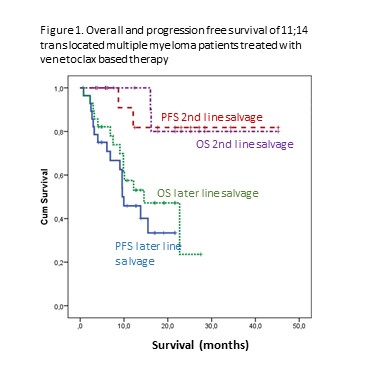
50 patients were reported from 7 Hungarian sites. 33 patients were relapsed and heavily pretreated with an average of 4.8 prior lines, whereas 17 patients received venetoclax after a suboptimal initial response to their first line treatment (8 PR, 7 SD, 2 PD) as planned pre-transplantation salvage. Most patients had venetoclax in combination with a proteasome inhibitor and dexamethasone. The response rate was remarkably high given the refractoriness of this cohort: all but two patients responded, with 28% CRs, 38% VGPRs and 30% PRs. The median PFS and OS calculated from initiation of venetoclax dosing were 15.5 and 24 months, respectively, Figure 1. shows the PFS and OS curves of the 2nd line and late line cohorts. The most common AEs were cytopenias, gastrointestinal toxicities and infections, reported in 8, 9 and 10 patients with 1 fatal infection. Two patients had COVID-19 related hospitalization, both recovered.
An important point to emphasize is the very high risk nature of this cohort. 13 out of the 47 patients had deletion 17p before venetoclax dosing was initiated, explaining their refractoriness to standard treatments. Notably, of these patients 2 reached CR, 9 VGPR, 1 PR, and only one progressed while on venetoclax treatment. The median PFS and OS were 9.6 and 10 months, respectively. Five patients had plasma cell leukaemia and 1 CNS involvement, the PFS and OS of these ultra-high risk patients were 10 and 12 months.
Another important aspect of our analysis was the question of venetoclax dosing, as the appropriate dose in this indication is not yet clear. Reflecting this uncertainty, as well as funding difficulties with this off-label drug, only 2 patients received the 800 mg dose as seen in the Bellini trial; one received 600 mg daily, with all others taking 400 mg or less. To counteract this many centers employed a combination with either clarithromycin or fluconazole, CYP3A inhibitors known to increase venetoclax serum levels two- to threefold. Where available, serum venetoclax levels were monitored to ensure serum levels comparable to regular dosing.
Conclusion
Our results highlight the importance of targeted treatments in multiple myeloma. We experienced lasting responses in quadruple-refractory patients, many with other high risk features. In the newly diagnosed group, where the depth of pre-ASCT response has great impact on PFS, venetoclax may have a role converting suboptimal responses into CRs by eliminating residual disease.
Keyword(s): BCL2, IgH translocation, Myeloma
Abstract: PB1641
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Clinical
Background
Venetoclax, a selective bcl-2 inhibitor first approved for CLL has been investigated for the treatment of relapsed myeloma patients, and although it failed to show benefit for myeloma patients as a whole, t(11;14) patients demonstrated exceptional responses in the Bellini trial (Lancet Oncol 2020; 21:1630), thus paving the way towards the first genetically targeted treatment in myeloma.
Aims
Off label use of venetoclax is on the rise, even though clinicians have to face unanswered questions regarding the right dosage and length of therapy, as well as the potential for adverse events (AEs), especially infections. Real world data could help to elucidate its optimal use, but is very limited as of yet.
Methods
We addressed all Hungarian centers treating myeloma to evaluate the efficacy and safety of venetoclax in their practice treating t(11;14) myeloma, collecting data about the treatment duration, AEs, dose modifications and treatment discontinuations, and analyzed response rates as well as progression free and overall survivals (PFS, OS).
Results
50 patients were reported from 7 Hungarian sites. 33 patients were relapsed and heavily pretreated with an average of 4.8 prior lines, whereas 17 patients received venetoclax after a suboptimal initial response to their first line treatment (8 PR, 7 SD, 2 PD) as planned pre-transplantation salvage. Most patients had venetoclax in combination with a proteasome inhibitor and dexamethasone. The response rate was remarkably high given the refractoriness of this cohort: all but two patients responded, with 28% CRs, 38% VGPRs and 30% PRs. The median PFS and OS calculated from initiation of venetoclax dosing were 15.5 and 24 months, respectively, Figure 1. shows the PFS and OS curves of the 2nd line and late line cohorts. The most common AEs were cytopenias, gastrointestinal toxicities and infections, reported in 8, 9 and 10 patients with 1 fatal infection. Two patients had COVID-19 related hospitalization, both recovered.
An important point to emphasize is the very high risk nature of this cohort. 13 out of the 47 patients had deletion 17p before venetoclax dosing was initiated, explaining their refractoriness to standard treatments. Notably, of these patients 2 reached CR, 9 VGPR, 1 PR, and only one progressed while on venetoclax treatment. The median PFS and OS were 9.6 and 10 months, respectively. Five patients had plasma cell leukaemia and 1 CNS involvement, the PFS and OS of these ultra-high risk patients were 10 and 12 months.
Another important aspect of our analysis was the question of venetoclax dosing, as the appropriate dose in this indication is not yet clear. Reflecting this uncertainty, as well as funding difficulties with this off-label drug, only 2 patients received the 800 mg dose as seen in the Bellini trial; one received 600 mg daily, with all others taking 400 mg or less. To counteract this many centers employed a combination with either clarithromycin or fluconazole, CYP3A inhibitors known to increase venetoclax serum levels two- to threefold. Where available, serum venetoclax levels were monitored to ensure serum levels comparable to regular dosing.

Conclusion
Our results highlight the importance of targeted treatments in multiple myeloma. We experienced lasting responses in quadruple-refractory patients, many with other high risk features. In the newly diagnosed group, where the depth of pre-ASCT response has great impact on PFS, venetoclax may have a role converting suboptimal responses into CRs by eliminating residual disease.
Keyword(s): BCL2, IgH translocation, Myeloma


