
Contributions
Abstract: PB1634
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Biology & Translational Research
Background
Multiple myeloma is a plasma cell malignancy and the second most common haematological cancer in Sweden. In vivo studies of myeloma in mice are usually done by injecting commercial cell lines intravenously. If we could successfully transplant treatment refractory human myeloma cells intratibially it would be a substantial benefit to test novel treatments and predict clinical outcome in patients. The bromodomain inhibitors (BETi), which inhibit reading of epigenetic modifications and block transcriptional activity, have sparked a lot of interest and showed promising results in animal studies of myeloma. An in-house compound under development called BETi-1 has shown promising results in vitro on commercial cell lines exhibiting at minimum equal potency as the widely-used compound JQ1 (data not yet published).
Aims
The primary objective of this project was to investigate whether it is possible to develop a patient derived xenograft (PDX) from human bone marrow aspirates of treatment refractory multiple myeloma. Secondary objectives were to examine if BETi affect the viability of myeloma cells in vivo.
Methods
In this translational study, biobank material consisting of cryopreserved bone marrow samples acquired from patients receiving treatments at Sahlgrenska University Hospital between 2017 and 2020 with relapsed/refractory multiple myeloma (RRMM), were thawed and transplanted intratibially on immunodeficient mice (NOD-SCID-IL2 receptor gamma knockout, NOG/NSG) to develop a patient-derived xenograft (PDX). Tumour progression was monitored with lambda- and kappa ELISA every other week. When progression was confirmed, the mice received either vehicle or treatment with BETi-1. At sacrifice, long bones and sternum were analysed with immunohistochemistry and FACS to verify presence of clonal plasma cells. All experiments were approved by the regional Ethical Committees in Gothenburg.
Results
We show a successfully established and serially transplanted myeloma PDX from human treatment refractory myeloma cells verified by two independent methods exhibiting clonal plasma cells with the ability to disseminate in NOG/NSG mice. In vivo experiment with BETi-1 treatment is still ongoing and will be presented in due time
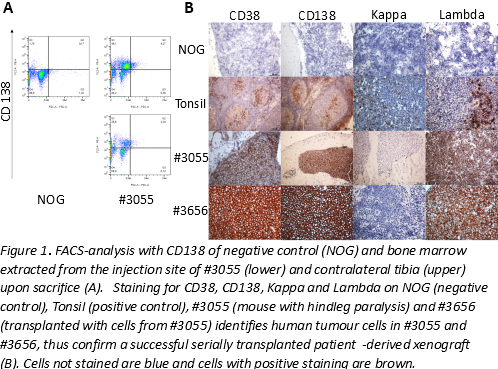
Conclusion
In this translational study we have established a multiple myeloma PDX model from human treatment refractory myeloma cells and thus developed a platform for examining new treatment options in multiple myeloma.
Keyword(s): Mouse model, Multiple myeloma, Refractory, Xenotransplantation
Abstract: PB1634
Type: Publication Only
Session title: Myeloma and other monoclonal gammopathies - Biology & Translational Research
Background
Multiple myeloma is a plasma cell malignancy and the second most common haematological cancer in Sweden. In vivo studies of myeloma in mice are usually done by injecting commercial cell lines intravenously. If we could successfully transplant treatment refractory human myeloma cells intratibially it would be a substantial benefit to test novel treatments and predict clinical outcome in patients. The bromodomain inhibitors (BETi), which inhibit reading of epigenetic modifications and block transcriptional activity, have sparked a lot of interest and showed promising results in animal studies of myeloma. An in-house compound under development called BETi-1 has shown promising results in vitro on commercial cell lines exhibiting at minimum equal potency as the widely-used compound JQ1 (data not yet published).
Aims
The primary objective of this project was to investigate whether it is possible to develop a patient derived xenograft (PDX) from human bone marrow aspirates of treatment refractory multiple myeloma. Secondary objectives were to examine if BETi affect the viability of myeloma cells in vivo.
Methods
In this translational study, biobank material consisting of cryopreserved bone marrow samples acquired from patients receiving treatments at Sahlgrenska University Hospital between 2017 and 2020 with relapsed/refractory multiple myeloma (RRMM), were thawed and transplanted intratibially on immunodeficient mice (NOD-SCID-IL2 receptor gamma knockout, NOG/NSG) to develop a patient-derived xenograft (PDX). Tumour progression was monitored with lambda- and kappa ELISA every other week. When progression was confirmed, the mice received either vehicle or treatment with BETi-1. At sacrifice, long bones and sternum were analysed with immunohistochemistry and FACS to verify presence of clonal plasma cells. All experiments were approved by the regional Ethical Committees in Gothenburg.
Results
We show a successfully established and serially transplanted myeloma PDX from human treatment refractory myeloma cells verified by two independent methods exhibiting clonal plasma cells with the ability to disseminate in NOG/NSG mice. In vivo experiment with BETi-1 treatment is still ongoing and will be presented in due time

Conclusion
In this translational study we have established a multiple myeloma PDX model from human treatment refractory myeloma cells and thus developed a platform for examining new treatment options in multiple myeloma.
Keyword(s): Mouse model, Multiple myeloma, Refractory, Xenotransplantation


