
Contributions
Abstract: PB1627
Type: Publication Only
Session title: Myelodysplastic syndromes - Clinical
Background
Low risk myelodysplastic syndromes (MDS) are a heterogeneous group of diseases that mainly present with anemia. The latter ranges from mild/asymptomatic to transfusion dependent and benefits from recombinant erythropoietin (rEPO) treatment in about 70% of cases. Several types of rEPO are available, and it is a matter of debate which one is the most effective and at what dose. Moreover, median duration of response is limited, and subsequent therapeutic options are scanty, so that most subjects would finally become transfusion dependent.
Aims
To evaluate the efficacy of an alternative rEPO product in patients with MDS refractory or relapsed after the first rEPO course.
Methods
MDS patients followed at two tertiary hematologic centers in Milan, Italy, subsequently treated with two different rEPO products have been included. Hematologic parameters, including endogenous EPO (eEPO) and transfusion dependence have been analysed before each rEPO course and response rates have been evaluated according to the International Working Group 2006 criteria.
Results
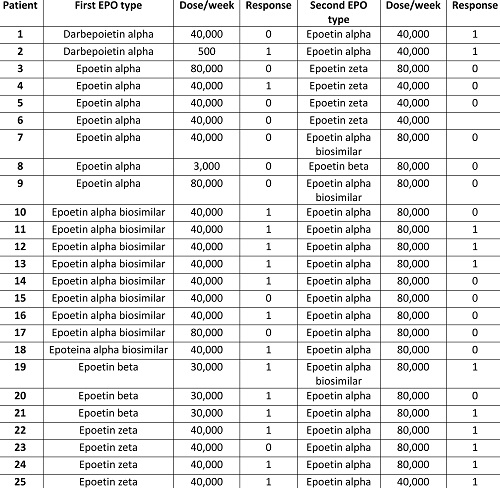
A total of 25 patients (14 males/15 females), with a median age of 74 years (59-85), followed for a median of 51 months (12-225) have been included. The majority suffered from MDS with ring sideroblasts (N=13) followed by MDS with multi-lineage dysplasia (N=7), single lineage dysplasia (N=2), 5q deletion (N=2), and excess of blasts type 1 (N=1); 15 cases had a low and 11 an intermediate-1 IPSS score. Bone marrow was hyper/normocellular in all cases but one, 10 patients showed reticulin fibrosis (MF-0), and 4 subjects harboured a cytogenetic aberration (including 5q and 11q23 deletions, and trisomy 8). Table 1 shows rEPO agents used and the relative doses/responses. Considering the first course, median eEPO pre-dose was 59 U/L (3-257) and 12 patients were transfusion dependent with a median need of 4 units/8 weeks (2-7). The first product utilized was mainly epoetin alpha (N=16, biosimilar in 9), followed by epoetin zeta (N=4), beta (N=3), and darbepoietin (N=2), with a median initial dose of 40,000 U/week, resulting in an overall response rate of 60% after 2.4 months (0.8-18). Median treatment duration was 20 months (2.4-81) with the first product, and patients were switched to an alternative compound due to loss of response (N=15), inefficacy (N=8), and toxicity (N=2). All had been increased to the maximal dose before switching. At the beginning of the second rEPO course 14 patients were transfusion dependant, and pre-dose eEPO was 142 U/L (43-390). Most patients started the alternative rEPO at high dose (80,000 U/w) and mainly shifted from alpha biosimilar to epoetin alpha (N=9) or vice versa (N=2), from alpha to zeta (N=4) or vice versa (N=4), from beta to alpha (N=3) or vice vera (N=1), and from darbepoietin to alpha (N=2). 44% of patients responded after a median of 1.9 months (0.7-5.2) from the switch, including 3 cases refractory to the first rEPO. Interestingly, 10/11 responders had been switched to epoetin alpha (p=0.03), 1 to the biosimilar, and only 27% were transfusion dependent before the switch (versus 78% in non-responders p=0.01). At the last follow up, 3 patients were still on rEPO, whilst 8 stopped it due to loss of response (N=6) or intolerance (N=2) after a median of 15.8 months (11.6-17.5).
Conclusion
Switching to an alternative rEPO was effective in 44% of cases, particularly in transfusion independent patients shifted to epoetin alpha. These results may suggest a try of an alternative rEPO product in both primary refractory MDS patients and in those relapsing after a first agent.
Keyword(s): Erythropoietin, Myelodysplasia
Abstract: PB1627
Type: Publication Only
Session title: Myelodysplastic syndromes - Clinical
Background
Low risk myelodysplastic syndromes (MDS) are a heterogeneous group of diseases that mainly present with anemia. The latter ranges from mild/asymptomatic to transfusion dependent and benefits from recombinant erythropoietin (rEPO) treatment in about 70% of cases. Several types of rEPO are available, and it is a matter of debate which one is the most effective and at what dose. Moreover, median duration of response is limited, and subsequent therapeutic options are scanty, so that most subjects would finally become transfusion dependent.
Aims
To evaluate the efficacy of an alternative rEPO product in patients with MDS refractory or relapsed after the first rEPO course.
Methods
MDS patients followed at two tertiary hematologic centers in Milan, Italy, subsequently treated with two different rEPO products have been included. Hematologic parameters, including endogenous EPO (eEPO) and transfusion dependence have been analysed before each rEPO course and response rates have been evaluated according to the International Working Group 2006 criteria.
Results
A total of 25 patients (14 males/15 females), with a median age of 74 years (59-85), followed for a median of 51 months (12-225) have been included. The majority suffered from MDS with ring sideroblasts (N=13) followed by MDS with multi-lineage dysplasia (N=7), single lineage dysplasia (N=2), 5q deletion (N=2), and excess of blasts type 1 (N=1); 15 cases had a low and 11 an intermediate-1 IPSS score. Bone marrow was hyper/normocellular in all cases but one, 10 patients showed reticulin fibrosis (MF-0), and 4 subjects harboured a cytogenetic aberration (including 5q and 11q23 deletions, and trisomy 8). Table 1 shows rEPO agents used and the relative doses/responses. Considering the first course, median eEPO pre-dose was 59 U/L (3-257) and 12 patients were transfusion dependent with a median need of 4 units/8 weeks (2-7). The first product utilized was mainly epoetin alpha (N=16, biosimilar in 9), followed by epoetin zeta (N=4), beta (N=3), and darbepoietin (N=2), with a median initial dose of 40,000 U/week, resulting in an overall response rate of 60% after 2.4 months (0.8-18). Median treatment duration was 20 months (2.4-81) with the first product, and patients were switched to an alternative compound due to loss of response (N=15), inefficacy (N=8), and toxicity (N=2). All had been increased to the maximal dose before switching. At the beginning of the second rEPO course 14 patients were transfusion dependant, and pre-dose eEPO was 142 U/L (43-390). Most patients started the alternative rEPO at high dose (80,000 U/w) and mainly shifted from alpha biosimilar to epoetin alpha (N=9) or vice versa (N=2), from alpha to zeta (N=4) or vice versa (N=4), from beta to alpha (N=3) or vice vera (N=1), and from darbepoietin to alpha (N=2). 44% of patients responded after a median of 1.9 months (0.7-5.2) from the switch, including 3 cases refractory to the first rEPO. Interestingly, 10/11 responders had been switched to epoetin alpha (p=0.03), 1 to the biosimilar, and only 27% were transfusion dependent before the switch (versus 78% in non-responders p=0.01). At the last follow up, 3 patients were still on rEPO, whilst 8 stopped it due to loss of response (N=6) or intolerance (N=2) after a median of 15.8 months (11.6-17.5).

Conclusion
Switching to an alternative rEPO was effective in 44% of cases, particularly in transfusion independent patients shifted to epoetin alpha. These results may suggest a try of an alternative rEPO product in both primary refractory MDS patients and in those relapsing after a first agent.
Keyword(s): Erythropoietin, Myelodysplasia


