
Contributions
Abstract: PB1626
Type: Publication Only
Session title: Myelodysplastic syndromes - Clinical
Background
Disease-related anemia presents a therapeutic challenge in patients (pts) with myelodysplastic syndromes (MDS) or multiple myeloma (MM). Regular red blood cell (RBC) transfusions in pts with MDS can lead to iron overload with potential clinical consequences, and one-third of pts with MM do not respond to erythropoietin-stimulating agents (ESAs). Activin receptor-like kinase-2 (ALK2) is an upstream regulator of hepcidin, the key regulatory hormone in iron homeostasis. Disrupted hepcidin homeostasis or elevated serum hepcidin levels can contribute to anemia in pts with MDS or MM. INCB000928, a selective ALK2 inhibitor, reduced hepcidin-induced anemia in preclinical models.
Aims
INCB 00928-105 (NCT04582539) is a phase 1/2, open-label, multicenter dose-escalation and dose-expansion study evaluating INCB000928 as monotherapy for pts with MDS- or MM-related anemia. The primary objective is to evaluate the safety and tolerability of INCB000928 monotherapy in pts with MDS or MM who present with transfusion-dependent or symptomatic anemia. Secondary objectives are to determine efficacy and pharmacokinetics of INCB000928, and effects of INCB000928 treatment on iron homeostasis and erythropoiesis parameters in pts with MDS or MM.
Methods
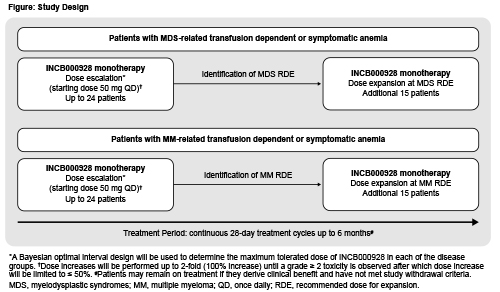
Pts aged ≥18 years with histologically confirmed MDS (or chronic myelomonocytic leukemia or unclassifiable MDS/myeloproliferative neoplasm [MPN] overlap syndrome) or MM who present with transfusion-dependent (RBC transfusion ≥4 units during 28 days pretreatment, or ≥4 units during 8 weeks pretreatment with hemoglobin [Hgb] <8.5 g/dL) or symptomatic anemia (baseline Hgb <10 g/dL on 3 separate occasions ≥7 days apart) are eligible to enroll. Other key inclusion criteria are: ineligibility for or lack of response to standard anemia treatments including ESAs; failure of standard MM treatments including alkylating agents, glucocorticoids, immunomodulators, proteasome inhibitors, daratumumab; ECOG PS ≤1 (dose-escalation) or ≤2 (dose-expansion); and life expectancy >6 months. Key exclusion criteria are: presence of MDS with ring sideroblasts or with atypical chronic myeloid leukemia, juvenile myelomonocytic leukemia, or MDS/MPN overlap syndromes with ring sideroblasts and thrombocytosis; MDS requiring cytoreductive treatment other than hydroxyurea; MDS with bone marrow and peripheral blood myeloblast count ≥10%; platelet count <50 × 109/L; or prior allogeneic stem cell transplantation. Treatment with oral INCB000928 monotherapy will start at 50 mg once daily in 28-day cycles (Figure). Dose escalation will follow a Bayesian optimal-interval design, independently and in parallel in each group, to determine the maximum tolerated doses; recommended doses for expansion (RDE) for each group will be based on safety and PK/PD data. Dose increases will be performed up to 2-fold until a dose-limiting toxicity is observed; subsequent increases will be limited to ≤50% of the prior dose. It is expected that up to 24 pts will be treated in each dose-escalation, and an additional 15 pts at the RDE in each dose-expansion. Pts will receive treatment for up to 6 months and may continue if deriving clinical benefit and no progression occurs. Sites are opening in the United States, France, and Italy.
Results
Not applicable
Conclusion
Not applicable
Keyword(s): Anemia, Kinase inhibitor, Multiple myeloma, Myeloproliferative disorder
Abstract: PB1626
Type: Publication Only
Session title: Myelodysplastic syndromes - Clinical
Background
Disease-related anemia presents a therapeutic challenge in patients (pts) with myelodysplastic syndromes (MDS) or multiple myeloma (MM). Regular red blood cell (RBC) transfusions in pts with MDS can lead to iron overload with potential clinical consequences, and one-third of pts with MM do not respond to erythropoietin-stimulating agents (ESAs). Activin receptor-like kinase-2 (ALK2) is an upstream regulator of hepcidin, the key regulatory hormone in iron homeostasis. Disrupted hepcidin homeostasis or elevated serum hepcidin levels can contribute to anemia in pts with MDS or MM. INCB000928, a selective ALK2 inhibitor, reduced hepcidin-induced anemia in preclinical models.
Aims
INCB 00928-105 (NCT04582539) is a phase 1/2, open-label, multicenter dose-escalation and dose-expansion study evaluating INCB000928 as monotherapy for pts with MDS- or MM-related anemia. The primary objective is to evaluate the safety and tolerability of INCB000928 monotherapy in pts with MDS or MM who present with transfusion-dependent or symptomatic anemia. Secondary objectives are to determine efficacy and pharmacokinetics of INCB000928, and effects of INCB000928 treatment on iron homeostasis and erythropoiesis parameters in pts with MDS or MM.
Methods
Pts aged ≥18 years with histologically confirmed MDS (or chronic myelomonocytic leukemia or unclassifiable MDS/myeloproliferative neoplasm [MPN] overlap syndrome) or MM who present with transfusion-dependent (RBC transfusion ≥4 units during 28 days pretreatment, or ≥4 units during 8 weeks pretreatment with hemoglobin [Hgb] <8.5 g/dL) or symptomatic anemia (baseline Hgb <10 g/dL on 3 separate occasions ≥7 days apart) are eligible to enroll. Other key inclusion criteria are: ineligibility for or lack of response to standard anemia treatments including ESAs; failure of standard MM treatments including alkylating agents, glucocorticoids, immunomodulators, proteasome inhibitors, daratumumab; ECOG PS ≤1 (dose-escalation) or ≤2 (dose-expansion); and life expectancy >6 months. Key exclusion criteria are: presence of MDS with ring sideroblasts or with atypical chronic myeloid leukemia, juvenile myelomonocytic leukemia, or MDS/MPN overlap syndromes with ring sideroblasts and thrombocytosis; MDS requiring cytoreductive treatment other than hydroxyurea; MDS with bone marrow and peripheral blood myeloblast count ≥10%; platelet count <50 × 109/L; or prior allogeneic stem cell transplantation. Treatment with oral INCB000928 monotherapy will start at 50 mg once daily in 28-day cycles (Figure). Dose escalation will follow a Bayesian optimal-interval design, independently and in parallel in each group, to determine the maximum tolerated doses; recommended doses for expansion (RDE) for each group will be based on safety and PK/PD data. Dose increases will be performed up to 2-fold until a dose-limiting toxicity is observed; subsequent increases will be limited to ≤50% of the prior dose. It is expected that up to 24 pts will be treated in each dose-escalation, and an additional 15 pts at the RDE in each dose-expansion. Pts will receive treatment for up to 6 months and may continue if deriving clinical benefit and no progression occurs. Sites are opening in the United States, France, and Italy.
Results
Not applicable

Conclusion
Not applicable
Keyword(s): Anemia, Kinase inhibitor, Multiple myeloma, Myeloproliferative disorder


