
Contributions
Abstract: PB1621
Type: Publication Only
Session title: Myelodysplastic syndromes - Clinical
Background
Myelodysplastic syndromes (LR-MDS) are a very heterogeneous group of diseases ranging from mild forms to life-treathening cytopenias. Recently, a deeper knowledge of LR MDS molecular landscape paved the way to novel treatments, such as luspatercept for SF3B1 mutated patients with ring sideroblasts. Less is known about the prevalence and type of other mutations in LR-MDS and their clinical significance.
Aims
To evaluate the prevalence of somatic mutations of myeloid genes in LR-MDS patients and their relationship with clinical/laboratory features and outcome.
Methods
Fifty out of 160 LR-MDS patients regularly followed at a tertiary hematologic center have been evaluated by next generation sequence (NGS) technology (Ion Torrents5) Ion Reporters software 5.2 which evaluates mutational status of 69 potentially oncogenic genes present in the Oncomine Myeloid Research Assay diagnostic panel, including hotspot genes, full genes, and fusion transcripts. Only variants with an allelic frequency (VAF) > 5% were reported. NGS results were matched with clinical and hematologic variables that have been retrospectively collected
Results
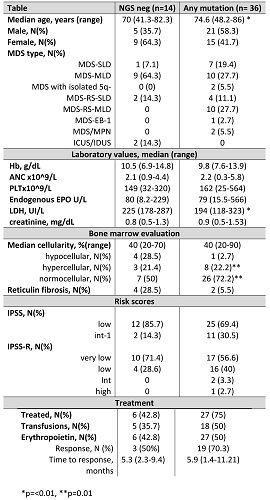
Table 1 shows the clinical features in mutated and unmutated patients. Seventy-two % of cases showed at least one mutation involving SF3B1 (N=16), TET2 (N=10), DNMT3A (N=4), SRSF2 (N=6), ASXL1 (N=5), ZRSR2 (N=3), IDH2 (N=3), IDH1 (N=2), SH2B3 (N=2), U2AF1 (N=4), P53 (N=1), ETV6 (N=1), PHF6 (N=2), STAG2 (N=1), RUNX1 (N=2), JAK2 V617F (N=2), FLT3 (N=1). The median VAF was 30%, and 15 patients showed 1 mutation, 10 patients 2 mutations, and 11 patients 3 or more. Subjects harbouring at least one mutation were older as compared to NGS negative cases (p<0.01), and more frequently displayed a normo/hypercellular bone marrow (p=0.01). Interestingly, mutated cases showed a higher response rate to recombinant erythropoietin (70.3% versus 50%), although not significantly. Subsequent analyses were made according to the number and type of genetic defects: harbouring 2 or more mutations was associated with older age [76 (41-86) versus 71 (48-85) years, p< 0.05], lower neutrophil counts at diagnosis [1.47 (0.4-4.2) versus 2.71 (0.4-5.8) x10^9/L, p<0.01], and to shorter time to response to recombinant EPO [2.2 (1.5-8.8) months versus 6.3 (1.5-16) months, p=0.06]. Neutropenia was even more marked in patients with 3 or more mutations [1.1 (0.2-2) versus 2.6 (1.9-5.2) x10^9/L, p<0.01]. Mutations involving the splicing pathway (i.e. SF3B1, U2AF1, and ZRSR2) were the most common, and correlated with older age (76 versus 71, p=0.01), increased bone marrow cellularity [50 (25-90) versus 40 (20-50)%, p<0.01], higher platelet [209 (41-564) versus 105 (20-332) x10^9/L, p=0.02] and neutrophil counts [2.8 (0.37-5.8 versus 1.8 (0.4-3.5) x10^9/L, p=0.03].
Conclusion
Somatic mutations involving myeloid genes were frequent in patients with LR-MDS and mainly correlated with older age and deeper cytopenias. The latter worsened along with the increase of mutations number. Considering mutation types, expectedly, those involving splicing pathway correlated with a more proliferative phenotype. Interestingly, somatic mutations did not negatively impact on response to recombinant erythropoietin
Keyword(s): Erythropoietin, Mutation analysis, Myelodysplasia
Abstract: PB1621
Type: Publication Only
Session title: Myelodysplastic syndromes - Clinical
Background
Myelodysplastic syndromes (LR-MDS) are a very heterogeneous group of diseases ranging from mild forms to life-treathening cytopenias. Recently, a deeper knowledge of LR MDS molecular landscape paved the way to novel treatments, such as luspatercept for SF3B1 mutated patients with ring sideroblasts. Less is known about the prevalence and type of other mutations in LR-MDS and their clinical significance.
Aims
To evaluate the prevalence of somatic mutations of myeloid genes in LR-MDS patients and their relationship with clinical/laboratory features and outcome.
Methods
Fifty out of 160 LR-MDS patients regularly followed at a tertiary hematologic center have been evaluated by next generation sequence (NGS) technology (Ion Torrents5) Ion Reporters software 5.2 which evaluates mutational status of 69 potentially oncogenic genes present in the Oncomine Myeloid Research Assay diagnostic panel, including hotspot genes, full genes, and fusion transcripts. Only variants with an allelic frequency (VAF) > 5% were reported. NGS results were matched with clinical and hematologic variables that have been retrospectively collected
Results
Table 1 shows the clinical features in mutated and unmutated patients. Seventy-two % of cases showed at least one mutation involving SF3B1 (N=16), TET2 (N=10), DNMT3A (N=4), SRSF2 (N=6), ASXL1 (N=5), ZRSR2 (N=3), IDH2 (N=3), IDH1 (N=2), SH2B3 (N=2), U2AF1 (N=4), P53 (N=1), ETV6 (N=1), PHF6 (N=2), STAG2 (N=1), RUNX1 (N=2), JAK2 V617F (N=2), FLT3 (N=1). The median VAF was 30%, and 15 patients showed 1 mutation, 10 patients 2 mutations, and 11 patients 3 or more. Subjects harbouring at least one mutation were older as compared to NGS negative cases (p<0.01), and more frequently displayed a normo/hypercellular bone marrow (p=0.01). Interestingly, mutated cases showed a higher response rate to recombinant erythropoietin (70.3% versus 50%), although not significantly. Subsequent analyses were made according to the number and type of genetic defects: harbouring 2 or more mutations was associated with older age [76 (41-86) versus 71 (48-85) years, p< 0.05], lower neutrophil counts at diagnosis [1.47 (0.4-4.2) versus 2.71 (0.4-5.8) x10^9/L, p<0.01], and to shorter time to response to recombinant EPO [2.2 (1.5-8.8) months versus 6.3 (1.5-16) months, p=0.06]. Neutropenia was even more marked in patients with 3 or more mutations [1.1 (0.2-2) versus 2.6 (1.9-5.2) x10^9/L, p<0.01]. Mutations involving the splicing pathway (i.e. SF3B1, U2AF1, and ZRSR2) were the most common, and correlated with older age (76 versus 71, p=0.01), increased bone marrow cellularity [50 (25-90) versus 40 (20-50)%, p<0.01], higher platelet [209 (41-564) versus 105 (20-332) x10^9/L, p=0.02] and neutrophil counts [2.8 (0.37-5.8 versus 1.8 (0.4-3.5) x10^9/L, p=0.03].

Conclusion
Somatic mutations involving myeloid genes were frequent in patients with LR-MDS and mainly correlated with older age and deeper cytopenias. The latter worsened along with the increase of mutations number. Considering mutation types, expectedly, those involving splicing pathway correlated with a more proliferative phenotype. Interestingly, somatic mutations did not negatively impact on response to recombinant erythropoietin
Keyword(s): Erythropoietin, Mutation analysis, Myelodysplasia


