
Contributions
Abstract: PB1613
Type: Publication Only
Session title: Myelodysplastic syndromes - Biology & Translational Research
Background
High-risk myelodysplastic syndrome (HR-MDS) patients have a short survival time and a poor prognosis. Moreover, there is dearth of therapeutic targets for treatment of this malignancy. Aldo-keto reductase AKR1B1 was reported to be highly expressed in a variety of tumors and promote the tumor cell proliferation.
Aims
The main objective was the identification of the role of the AKR1B1 axis in the progression of HR-MDS and its druggable potential
Methods
Kaplan Meier curve was used to evaluate the relationship of AKR1B1 expression level and overall survival in HR-MDS. The effect of AKR1B1 knockdown and overexpression and the effect of AKR1B1 inhibitor epalrestat was studied in cell viability. To evaluate the combined effect of epalrestat and hypomethylating agents (HMAs), data were analyzed by the CalcuSyn software. The related signaling pathways of AKR1B1 in MDS were investigated by gene set enrichment analysis (GSEA) and verified by Western blot. Co-immunoprecipitation (Co-IP) was performed to detect proteins that interact with AKR1B1.
Results
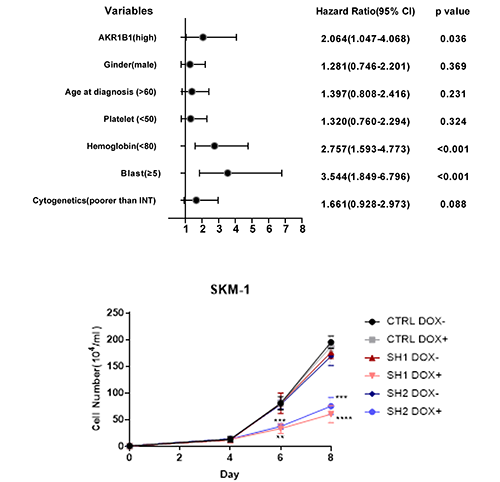
AKR1B1 was upregulated in high-risk MDS, not low-risk MDS. AKR1B1 expression was increased in EZH2 mutant MDS patients. In the hematopoietic stem and progenitor cells (HSPCs), AKR1B1 was barely expressed. High expression of AKR1B1 was associated with shorter OS and EFS in HR-MDS. Multivariate analysis revealed that higher AKR1B1 expression was an independent, unfavourable prognostic factor for OS in HR-MDS, not in the total MDS.
Genetic or pharmacological inhibition of AKR1B1 markedly decreased cell viability of MDS cell lines. Similar results were obtained in primary MDS cells. SKM-1 cells overexpressing AKR1B1 had a higher proliferation rate under serum starvation. Epalrestat enhances the toxicity of HMA in MDS cell line and primary MDS cells.
GSEA results suggested that AKR1B1 might participate in the mTORC1 signaling pathway, which was confirmed by Westernblot. Mechanistically, AKR1B1 interacted with RHEB to regulate the phosphorylation of mTOR.
Conclusion
AKR1B1 is negatively correlated with overall survival in HR-MDS. Inhibition of AKR1B1 not only suppressed the proliferation of MDS cells but also increase the drug susceptibility to HMAs. AKR1B1 interacts with RHEB to modulate mTORC1 activity. In conclusion, suppression of AKR1B1 reduces cell proliferation and enhances sensitivity to HMAs by inhibiting mTORC1 signaling pathway in HR-MDS.
Keyword(s): MDS, MTOR, Proliferation
Abstract: PB1613
Type: Publication Only
Session title: Myelodysplastic syndromes - Biology & Translational Research
Background
High-risk myelodysplastic syndrome (HR-MDS) patients have a short survival time and a poor prognosis. Moreover, there is dearth of therapeutic targets for treatment of this malignancy. Aldo-keto reductase AKR1B1 was reported to be highly expressed in a variety of tumors and promote the tumor cell proliferation.
Aims
The main objective was the identification of the role of the AKR1B1 axis in the progression of HR-MDS and its druggable potential
Methods
Kaplan Meier curve was used to evaluate the relationship of AKR1B1 expression level and overall survival in HR-MDS. The effect of AKR1B1 knockdown and overexpression and the effect of AKR1B1 inhibitor epalrestat was studied in cell viability. To evaluate the combined effect of epalrestat and hypomethylating agents (HMAs), data were analyzed by the CalcuSyn software. The related signaling pathways of AKR1B1 in MDS were investigated by gene set enrichment analysis (GSEA) and verified by Western blot. Co-immunoprecipitation (Co-IP) was performed to detect proteins that interact with AKR1B1.
Results
AKR1B1 was upregulated in high-risk MDS, not low-risk MDS. AKR1B1 expression was increased in EZH2 mutant MDS patients. In the hematopoietic stem and progenitor cells (HSPCs), AKR1B1 was barely expressed. High expression of AKR1B1 was associated with shorter OS and EFS in HR-MDS. Multivariate analysis revealed that higher AKR1B1 expression was an independent, unfavourable prognostic factor for OS in HR-MDS, not in the total MDS.
Genetic or pharmacological inhibition of AKR1B1 markedly decreased cell viability of MDS cell lines. Similar results were obtained in primary MDS cells. SKM-1 cells overexpressing AKR1B1 had a higher proliferation rate under serum starvation. Epalrestat enhances the toxicity of HMA in MDS cell line and primary MDS cells.
GSEA results suggested that AKR1B1 might participate in the mTORC1 signaling pathway, which was confirmed by Westernblot. Mechanistically, AKR1B1 interacted with RHEB to regulate the phosphorylation of mTOR.

Conclusion
AKR1B1 is negatively correlated with overall survival in HR-MDS. Inhibition of AKR1B1 not only suppressed the proliferation of MDS cells but also increase the drug susceptibility to HMAs. AKR1B1 interacts with RHEB to modulate mTORC1 activity. In conclusion, suppression of AKR1B1 reduces cell proliferation and enhances sensitivity to HMAs by inhibiting mTORC1 signaling pathway in HR-MDS.
Keyword(s): MDS, MTOR, Proliferation


