
Contributions
Abstract: PB1606
Type: Publication Only
Session title: Iron metabolism, deficiency and overload
Background
HFE hemochromatosis is characterized by iron overload due to increased iron absorption. Serum ferritin (SF) reflects total body storage iron, and the combination of elevated SF levels and an elevated transferrin saturation (Tsat) offers an easy and inexpensive screening method to detect candidates for HFE genetic testing. Overt disease is mainly associated with the C282Y homozygote genotype, and recommendations regarding phlebotomy to achieve iron depletion in these patients are well established. Fewer studies have focused on SF levels and its association with iron overload in other HFE genotypes, and accordingly to the controversy regarding risk of a significant iron overload developing in non-C282Y homozygotes, recommendations regarding management of these patients differ.
Aims
Based on these considerations, we wanted to investigate distribution and phenotypic expression of C282Y and H63D HFE mutations in patients referred to first time therapeutic phlebotomy, based on clinical suspicion of hemochromatosis or biochemical findings indicating iron overload.
Methods
We conducted a monocenter, cross-sectional study and retrospectively reviewed hospital records of patients who had positive genetic testing for C282Y and/or H63D HFE mutations over a 7-year period, and who were referred by their general practitioner to a tertiary referral center upon suspected hereditary hemochromatosis. 431 men and 97 women referred to first time therapeutic phlebotomy by the treating physician were included in the final analysis. Age at referral and last set of laboratory parameters prior to initiation of phlebotomy were obtained, including the following: SF, Tsat, hemoglobin (Hb), leukocyte particle concentration (LPK), thrombocyte particle concentration (TPK) and C-reactive protein (CRP).
Results
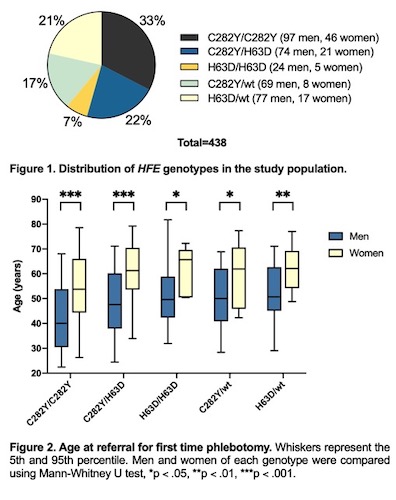
Median age at referral was 47.8 years in men and 57.1 years in women (p < .001). C282Y homozygosity was the most prevalent while H63D homozygosity was the least prevalent genotype in both genders (fig. 1). C282Y homozygotes represented the youngest group in both men (median 40.1 years, range 18.9 to 76.8) and women (median 53.8 years, range 25.1 to 79.5) (p < .001) (fig. 2). In men, H63D heterozygotes had the highest age at referral (median 50.7 years), while H63D homozygotes showed the highest age at referral among women (median 65.7 years). Finally, SF levels were lower in women (median 625 μg/L) than men (median 706 μg/L) (p < .001).
Only 10.8% of men and 3.8% of women exhibited SF levels ≥ 1000 μg/L in combination with Tsat level ≥ 50%. C282Y homozygosity was the most prevalent genotype of this group, although other genotypes were also represented. Additionally, 37% of men and 16% of women exhibiting Tsat levels ≥ 50% were non-C282Y homozygotes.
Conclusion
Our results are in line with previous observations regarding elevated iron indices compatible with iron overload not only in the C282Y homozygous state, but also in other HFE genotypes. Clinical practice regarding therapeutic phlebotomy in non-C282Y homozygotes is often restrictive, as risk of complications due to iron overload in these individuals is generally considered as low. Nevertheless, iron overload is not independently dependent on HFE mutations status, as environmental and host risk factors may modulate expression and contribute to a variable clinical presentation. Hence, phlebotomy ought to be considered in non-C282Y homozygote patients with persistent hyperferritinemia and suspected iron overload, particularly when other risk factors are present and further assessment is unwarranted.
Keyword(s): Ferritin, Hemochromatosis, Iron overload
Abstract: PB1606
Type: Publication Only
Session title: Iron metabolism, deficiency and overload
Background
HFE hemochromatosis is characterized by iron overload due to increased iron absorption. Serum ferritin (SF) reflects total body storage iron, and the combination of elevated SF levels and an elevated transferrin saturation (Tsat) offers an easy and inexpensive screening method to detect candidates for HFE genetic testing. Overt disease is mainly associated with the C282Y homozygote genotype, and recommendations regarding phlebotomy to achieve iron depletion in these patients are well established. Fewer studies have focused on SF levels and its association with iron overload in other HFE genotypes, and accordingly to the controversy regarding risk of a significant iron overload developing in non-C282Y homozygotes, recommendations regarding management of these patients differ.
Aims
Based on these considerations, we wanted to investigate distribution and phenotypic expression of C282Y and H63D HFE mutations in patients referred to first time therapeutic phlebotomy, based on clinical suspicion of hemochromatosis or biochemical findings indicating iron overload.
Methods
We conducted a monocenter, cross-sectional study and retrospectively reviewed hospital records of patients who had positive genetic testing for C282Y and/or H63D HFE mutations over a 7-year period, and who were referred by their general practitioner to a tertiary referral center upon suspected hereditary hemochromatosis. 431 men and 97 women referred to first time therapeutic phlebotomy by the treating physician were included in the final analysis. Age at referral and last set of laboratory parameters prior to initiation of phlebotomy were obtained, including the following: SF, Tsat, hemoglobin (Hb), leukocyte particle concentration (LPK), thrombocyte particle concentration (TPK) and C-reactive protein (CRP).
Results
Median age at referral was 47.8 years in men and 57.1 years in women (p < .001). C282Y homozygosity was the most prevalent while H63D homozygosity was the least prevalent genotype in both genders (fig. 1). C282Y homozygotes represented the youngest group in both men (median 40.1 years, range 18.9 to 76.8) and women (median 53.8 years, range 25.1 to 79.5) (p < .001) (fig. 2). In men, H63D heterozygotes had the highest age at referral (median 50.7 years), while H63D homozygotes showed the highest age at referral among women (median 65.7 years). Finally, SF levels were lower in women (median 625 μg/L) than men (median 706 μg/L) (p < .001).
Only 10.8% of men and 3.8% of women exhibited SF levels ≥ 1000 μg/L in combination with Tsat level ≥ 50%. C282Y homozygosity was the most prevalent genotype of this group, although other genotypes were also represented. Additionally, 37% of men and 16% of women exhibiting Tsat levels ≥ 50% were non-C282Y homozygotes.

Conclusion
Our results are in line with previous observations regarding elevated iron indices compatible with iron overload not only in the C282Y homozygous state, but also in other HFE genotypes. Clinical practice regarding therapeutic phlebotomy in non-C282Y homozygotes is often restrictive, as risk of complications due to iron overload in these individuals is generally considered as low. Nevertheless, iron overload is not independently dependent on HFE mutations status, as environmental and host risk factors may modulate expression and contribute to a variable clinical presentation. Hence, phlebotomy ought to be considered in non-C282Y homozygote patients with persistent hyperferritinemia and suspected iron overload, particularly when other risk factors are present and further assessment is unwarranted.
Keyword(s): Ferritin, Hemochromatosis, Iron overload


