
Contributions
Abstract: PB1603
Type: Publication Only
Session title: Iron metabolism, deficiency and overload
Background
Iron Deficiency Anemia (IDA) is a common health problem in daily clinical practice. A better understanding of iron metabolism in recent years; has created the need for revising treatment regimens in the treatment of IDA.
Aims
Based on these data, we aimed to evaluate the effectiveness of oral ferrous sulfate treatment at different doses, and posologies in premenopausal women diagnosed with IDA, and its relationship with hepcidin, treatment compliance, and gastrointestinal side effects in this study.
Methods
This is a prospective observational study including premenopausal female patients median age 36 (18-50) diagnosed with IDA. This study was approved by local ethic committe and sponsored by Ankara University School of Medicine Scientific Research Projects(BAP proje number 20L0230012) All patients recieved po ferrous sulfate (FS) 80 mg elemental iron) for three months. Patients were classified into three group according to FS dose: Group 1: 2x1/day; Group 2: 1x1/day; Group 3: 1x1every other day. At the time of enrollment, thereafter at the second week of FS and at the third month: Hb level, transferrin saturation( %TS), total iron binding capacity were recorded. Blood was drawn and stored at -20 temperature for hepcidin analysis from patiens who signed informed consent.
Results
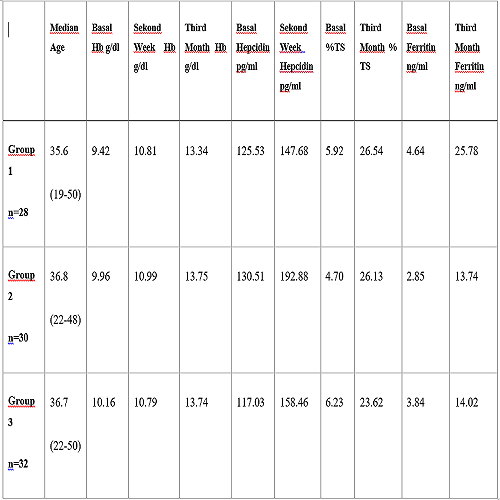
We intent to analyse 50 patients for each group, however as a consequence of COVID-19 pandemic, neither patients could come to hospitals nor we could visit them. The patients in each group, ages and results are shown Table At the end of the second week a significant increase in Hb level was observed (p <0.01) and the mean Hb level increased by 1.38 ± 1.04 and 1.03 ± 0.48 in the first and second groups respectively and ≥ 1 g / dl while in the group given every other day it remained <1 g / dl with an increase of 0.69 ± 0.36. (p = 0.020, p = 0.019, respectively). Comparing the ferritin levels within the three group, it was seen that the ferritin increased significantly in the first group compared to the second and third group (p <0.05). There was no difference between group 2 and group 3 in terms of increase in ferritin level (p> 0.05). The increase in TS% and decrease in total iron binding capacity were similar in all three group at the end of the treatment (p> 0.05). The change between the second week and the initial hepcidin was mostly observed in the second group (p= 0.024) and the change between the three group were similar (p = 0.708). Gastrointestinal side effects were observed more in the first group who received 2*1 daily than in the second and third group (p <0.05). While a similar increase in appetite and weight was observed in the patients in group 1 and 2 at the end of the treatment (p> 0.05) and there was no increase in appetite and weight in group 3.
Conclusion
Hb level increase at the end of second week was ≥1 g / dl in the first and second group and <1 g / dl in the third treatment group. However, at the end of the treatment third month anemia was significantly improved in all three group and the increase in Hb level was similar. We consider, 1*1 daily or 1*1 every other day posology would be more appropriate instead of 2*1 daily due to the significant gastrointestinal side effects in the first group . In addition, we believe in that, the evaluation of Hb response in the second week is too early to decide treatment outcome due to the slower Hb increase in the other day group and serial Hepcidin measurements will give a better idea to better understand the kinetics.
Keyword(s): Hepcidin, Iron deficiency anemia
Abstract: PB1603
Type: Publication Only
Session title: Iron metabolism, deficiency and overload
Background
Iron Deficiency Anemia (IDA) is a common health problem in daily clinical practice. A better understanding of iron metabolism in recent years; has created the need for revising treatment regimens in the treatment of IDA.
Aims
Based on these data, we aimed to evaluate the effectiveness of oral ferrous sulfate treatment at different doses, and posologies in premenopausal women diagnosed with IDA, and its relationship with hepcidin, treatment compliance, and gastrointestinal side effects in this study.
Methods
This is a prospective observational study including premenopausal female patients median age 36 (18-50) diagnosed with IDA. This study was approved by local ethic committe and sponsored by Ankara University School of Medicine Scientific Research Projects(BAP proje number 20L0230012) All patients recieved po ferrous sulfate (FS) 80 mg elemental iron) for three months. Patients were classified into three group according to FS dose: Group 1: 2x1/day; Group 2: 1x1/day; Group 3: 1x1every other day. At the time of enrollment, thereafter at the second week of FS and at the third month: Hb level, transferrin saturation( %TS), total iron binding capacity were recorded. Blood was drawn and stored at -20 temperature for hepcidin analysis from patiens who signed informed consent.
Results
We intent to analyse 50 patients for each group, however as a consequence of COVID-19 pandemic, neither patients could come to hospitals nor we could visit them. The patients in each group, ages and results are shown Table At the end of the second week a significant increase in Hb level was observed (p <0.01) and the mean Hb level increased by 1.38 ± 1.04 and 1.03 ± 0.48 in the first and second groups respectively and ≥ 1 g / dl while in the group given every other day it remained <1 g / dl with an increase of 0.69 ± 0.36. (p = 0.020, p = 0.019, respectively). Comparing the ferritin levels within the three group, it was seen that the ferritin increased significantly in the first group compared to the second and third group (p <0.05). There was no difference between group 2 and group 3 in terms of increase in ferritin level (p> 0.05). The increase in TS% and decrease in total iron binding capacity were similar in all three group at the end of the treatment (p> 0.05). The change between the second week and the initial hepcidin was mostly observed in the second group (p= 0.024) and the change between the three group were similar (p = 0.708). Gastrointestinal side effects were observed more in the first group who received 2*1 daily than in the second and third group (p <0.05). While a similar increase in appetite and weight was observed in the patients in group 1 and 2 at the end of the treatment (p> 0.05) and there was no increase in appetite and weight in group 3.

Conclusion
Hb level increase at the end of second week was ≥1 g / dl in the first and second group and <1 g / dl in the third treatment group. However, at the end of the treatment third month anemia was significantly improved in all three group and the increase in Hb level was similar. We consider, 1*1 daily or 1*1 every other day posology would be more appropriate instead of 2*1 daily due to the significant gastrointestinal side effects in the first group . In addition, we believe in that, the evaluation of Hb response in the second week is too early to decide treatment outcome due to the slower Hb increase in the other day group and serial Hepcidin measurements will give a better idea to better understand the kinetics.
Keyword(s): Hepcidin, Iron deficiency anemia


