
Contributions
Abstract: PB1601
Type: Publication Only
Session title: Iron metabolism, deficiency and overload
Background
Persistently raised hemoglobin (Hb) and hematocrit (Ht) levels characterize Erythrocytosis which cause in some cases is not recognized despite a careful study. In these patients a diagnosis of Idiopathic Erythrocytosis (IE) is carried out.
Recent observations suggest that some IE patients carry mutations or polymorphisms of HFE gene. This gene is involved in the Iron Metabolism regulation and these data suggest that some other cases of IE may be due to an alteration of Iron Mechanism involved genes.
Aims
We constructed a genes panel for Next Generation Sequencing (NGS) to search mutations of already known genes involved in Erythrocytosis and in those involved in Iron Metabolism in patients with increased Ht of not defined origin.
Methods
Fifteen genes composed the panel designed for NGS: JAK2, EGLN1 (PHD2), EPOR, FTL, FTH, ASXL1, HFE, HFE2, TFR2, HAMP, SLC40A1, SLC11A2, VHL, BPMG, EPAS1. All the exons part of the 15 genes were analyzed. The approach used for the library preparation was a multiplexing PCR and data were analyzed by bioinformatics tools. In all patients, the mutations found were confirmed with Sanger Sequencing.
Results
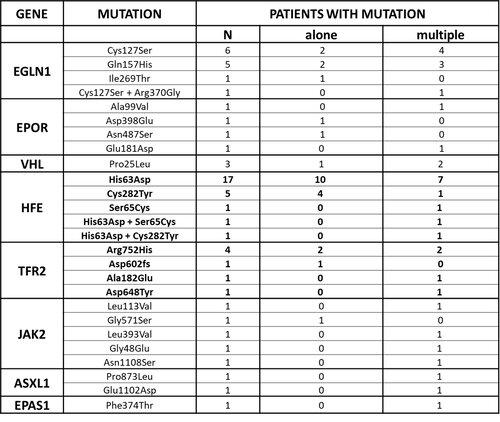
We studied 68 patients with erythrocytosis not carrying any JAK2 somatic mutation. We found 44 patients carrying at least one germline mutation in the genes of the panel. The germline mutations found in this study are reported in Table 1: 17 interest oxygen sensing pathway, 4 EPOR, 2 ASXL1 and 5 JAK2 genes. Moreover, we found 25 patients with at least one missense mutation in HFE gene and 7 patients with a mutation in TFR2 gene.
Conclusion
Our data show the validity of the chosen NGS panel. The use of this panel allowed us to found mutations already known to be related to erythrocytosis, and also to find some cases characterized by mutations of iron metabolism genes, as we previously found with sequencing method. The NGS genes panel evaluated offers the possibility to easily recognize new mutations explaining IE. The researchers have to consider iron metabolism to be possibly involved in the etiology of IE.
Keyword(s): Erythrocytosis, Gene polymorphism, Genetic
Abstract: PB1601
Type: Publication Only
Session title: Iron metabolism, deficiency and overload
Background
Persistently raised hemoglobin (Hb) and hematocrit (Ht) levels characterize Erythrocytosis which cause in some cases is not recognized despite a careful study. In these patients a diagnosis of Idiopathic Erythrocytosis (IE) is carried out.
Recent observations suggest that some IE patients carry mutations or polymorphisms of HFE gene. This gene is involved in the Iron Metabolism regulation and these data suggest that some other cases of IE may be due to an alteration of Iron Mechanism involved genes.
Aims
We constructed a genes panel for Next Generation Sequencing (NGS) to search mutations of already known genes involved in Erythrocytosis and in those involved in Iron Metabolism in patients with increased Ht of not defined origin.
Methods
Fifteen genes composed the panel designed for NGS: JAK2, EGLN1 (PHD2), EPOR, FTL, FTH, ASXL1, HFE, HFE2, TFR2, HAMP, SLC40A1, SLC11A2, VHL, BPMG, EPAS1. All the exons part of the 15 genes were analyzed. The approach used for the library preparation was a multiplexing PCR and data were analyzed by bioinformatics tools. In all patients, the mutations found were confirmed with Sanger Sequencing.
Results
We studied 68 patients with erythrocytosis not carrying any JAK2 somatic mutation. We found 44 patients carrying at least one germline mutation in the genes of the panel. The germline mutations found in this study are reported in Table 1: 17 interest oxygen sensing pathway, 4 EPOR, 2 ASXL1 and 5 JAK2 genes. Moreover, we found 25 patients with at least one missense mutation in HFE gene and 7 patients with a mutation in TFR2 gene.

Conclusion
Our data show the validity of the chosen NGS panel. The use of this panel allowed us to found mutations already known to be related to erythrocytosis, and also to find some cases characterized by mutations of iron metabolism genes, as we previously found with sequencing method. The NGS genes panel evaluated offers the possibility to easily recognize new mutations explaining IE. The researchers have to consider iron metabolism to be possibly involved in the etiology of IE.
Keyword(s): Erythrocytosis, Gene polymorphism, Genetic


