
Contributions
Abstract: PB1576
Type: Publication Only
Session title: Indolent and mantle-cell non-Hodgkin lymphoma - Clinical
Background
Major salivary gland marginal zone lymphoma (MSGMZL) is a rare malignant tumor with predominantly early-stage (I/II) lesions.
Aims
This study aims to identify prognostic factors in early-stage MSGMZL and compare long-term outcomes of conventional approaches.
Methods
We conducted a population-based cohort study utilizing the Surveillance Epidemiology and End Results (SEER) database and used log-rank test, Cox regression, and propensity score matching (PSM) analyses to explore the association of characteristics and therapeutic methods with survival.
Results
We identified 1,091 early-stage MSGMZL cases with a median follow-up of 81.0 months. Univariate and multivariate analyses indicated that age over 60 years at diagnosis, diagnosis before 2005, and coexisting malignancy were unfavorable independent predictors for overall survival; the former two also were adverse predictors for disease-specific survival. By PSM, we compared the effects of conventional interventions, including surgery, radiotherapy, and chemotherapy, and found no statistically significant differences between treatments after matching. In the subgroup analysis according to the above prognostic factors, there was inadequate data to identify any one of the conventional therapies as superior to the others. A notable one is patients diagnosed after 2005 had better survival curves than those diagnosed before 2005 with the same strategy, indicating that there might other therapy involved in the treatment of patients with early-stage MSGMZL. According to the literature, the anti-CD20 monoclonal antibody is the most promising drug responsible for improved survival.
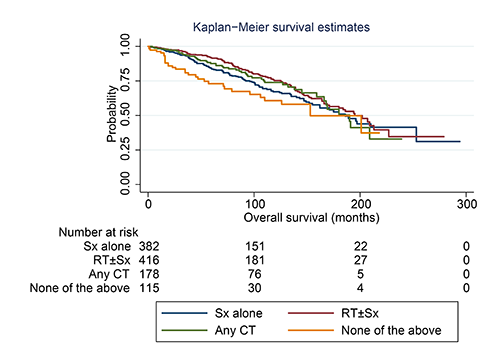
Conclusion
In this retrospective study from a database, conventional interventions failed to provide any survival benefit for early-stage MSGMZL patients. A novel therapy might be a better alternative.
Keyword(s): Lymphoma therapy, Marginal zone, Survival
Abstract: PB1576
Type: Publication Only
Session title: Indolent and mantle-cell non-Hodgkin lymphoma - Clinical
Background
Major salivary gland marginal zone lymphoma (MSGMZL) is a rare malignant tumor with predominantly early-stage (I/II) lesions.
Aims
This study aims to identify prognostic factors in early-stage MSGMZL and compare long-term outcomes of conventional approaches.
Methods
We conducted a population-based cohort study utilizing the Surveillance Epidemiology and End Results (SEER) database and used log-rank test, Cox regression, and propensity score matching (PSM) analyses to explore the association of characteristics and therapeutic methods with survival.
Results
We identified 1,091 early-stage MSGMZL cases with a median follow-up of 81.0 months. Univariate and multivariate analyses indicated that age over 60 years at diagnosis, diagnosis before 2005, and coexisting malignancy were unfavorable independent predictors for overall survival; the former two also were adverse predictors for disease-specific survival. By PSM, we compared the effects of conventional interventions, including surgery, radiotherapy, and chemotherapy, and found no statistically significant differences between treatments after matching. In the subgroup analysis according to the above prognostic factors, there was inadequate data to identify any one of the conventional therapies as superior to the others. A notable one is patients diagnosed after 2005 had better survival curves than those diagnosed before 2005 with the same strategy, indicating that there might other therapy involved in the treatment of patients with early-stage MSGMZL. According to the literature, the anti-CD20 monoclonal antibody is the most promising drug responsible for improved survival.

Conclusion
In this retrospective study from a database, conventional interventions failed to provide any survival benefit for early-stage MSGMZL patients. A novel therapy might be a better alternative.
Keyword(s): Lymphoma therapy, Marginal zone, Survival


