
Contributions
Abstract: PB1573
Type: Publication Only
Session title: Indolent and mantle-cell non-Hodgkin lymphoma - Clinical
Background
Bendamustine plus rituximab (B+R) was established as a preferred first line therapy for patients with previously untreated indolent non-Hodgkin’s lymphoma based on the BRIGHT and STIL trials. However, only few reports on efficacy and safety data of this combination in the real-world setting are available to-date.
Aims
To assess pattern of bendamustine + rituximab administration, efficacy and safety in non-clinical trial setting.
Methods
We conducted a retrospective review of patients who received therapy with B+R in our cancer center from June 2013 to dECEMBER 31, 2020. Patients with indolent non-Hodgkin’s lymphoma (iNHL) and mantle cell lymphoma (MCL) who received at least one cycle of B+R were evaluated.
Results
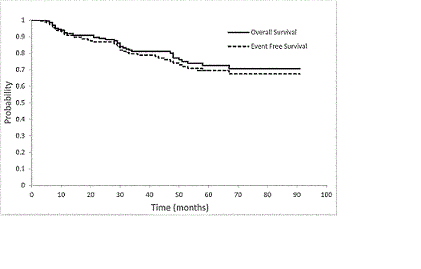
Amongst a total of 201 patients 56% were males and 44% females. Median age at B+R initiation was 72 years (range 34-94). Follicular lymphoma (FL) (50.3%), marginal zone lymphoma (MZL) (19.4%), and lymphoplasmacytic lymphoma (LPL) (14.5%) were the most common iNHL. Stage 3 and 4 diseases represented 19.9% and 68.6% of patients. Extranodal disease was found in 35.8%. The proportion of patients with high-risk disease was 48.5% for FL (FLIPI score ≥3), 86.6% for LPL (WMISS score ≥2), and 80.5% for MCL (MIPI score ≥6.2). History of secondary malignancy was presented in 23.4% of patients; 88.6% had ECOG 1-2, and 11.4% ECOG 3. Most common indications for B+R initiation were bulky symptomatic lymphadenopathy (69.1%), cytopenia (36.8%) and constitutional symptoms (36.8%). Fifty-eight percent of patients had more than one indication for therapy. Median number of B+R cycles delivered was 6 (range: 1-6), median dose of bendamustine was 90 mg/m2 (range: 45-90). Full doses of bendamustine were given in 66.7% of patients. In patients (33.3%) with dose reduction, the mean dose was 78.3 mg/m2. A total of 50.8% completed 6 cycles with no delays; and 49.2% of treatment was delayed (mean delay time :1.8 weeks). Overall response was 94.5%, with 77.1% complete and 17.4% partial remission. Median follow-up duration was 35 months (range: 4-91). At the end of follow-up, event free survival (EFS) was 77.1% and overall survival (OS) was 79.6%. Six percent of patients relapsed. In total, 14.5% develop secondary malignancies (6.5% non-hematological and 8% hematological) including 14 cases (7%) of aggressive B-cell lymphoma and 2 cases of myelodysplastic syndroms. A total of 16.9% of patients required support with G-CSF. The total number of adverse events was the highest in cycle one and decreased consistently with each cycle. Grade 3-4 neutropenia was recorded in 17.4%, febrile neutropenia in 8.0%, grade 3-4 anemia in 8.0%, and grade 3-4 thrombocytopenia in 3.9% of patients. Rituximab-associated infusion reactions were the most common in cycle 1 (46%) and 2 (16%). The rate of skin rash, fatigue, nausea/vomiting and infections were similar in each cycle. A total of 84.6% of patients proceeded to rituximab maintenance.
Conclusion
B+R chemoimmunotherapy was feasible to administer and retained its efficacy in non-clinical trial (real world) setting. HIgehr than previously reported rate of aggressive B-cell lymphoma was noted. No new adverse events of secondary non-hematological malignancies were found.
Keyword(s): Bendamustine, Mantle cell lymphoma, Non-Hodgkin's lymphoma, Rituximab
Abstract: PB1573
Type: Publication Only
Session title: Indolent and mantle-cell non-Hodgkin lymphoma - Clinical
Background
Bendamustine plus rituximab (B+R) was established as a preferred first line therapy for patients with previously untreated indolent non-Hodgkin’s lymphoma based on the BRIGHT and STIL trials. However, only few reports on efficacy and safety data of this combination in the real-world setting are available to-date.
Aims
To assess pattern of bendamustine + rituximab administration, efficacy and safety in non-clinical trial setting.
Methods
We conducted a retrospective review of patients who received therapy with B+R in our cancer center from June 2013 to dECEMBER 31, 2020. Patients with indolent non-Hodgkin’s lymphoma (iNHL) and mantle cell lymphoma (MCL) who received at least one cycle of B+R were evaluated.
Results
Amongst a total of 201 patients 56% were males and 44% females. Median age at B+R initiation was 72 years (range 34-94). Follicular lymphoma (FL) (50.3%), marginal zone lymphoma (MZL) (19.4%), and lymphoplasmacytic lymphoma (LPL) (14.5%) were the most common iNHL. Stage 3 and 4 diseases represented 19.9% and 68.6% of patients. Extranodal disease was found in 35.8%. The proportion of patients with high-risk disease was 48.5% for FL (FLIPI score ≥3), 86.6% for LPL (WMISS score ≥2), and 80.5% for MCL (MIPI score ≥6.2). History of secondary malignancy was presented in 23.4% of patients; 88.6% had ECOG 1-2, and 11.4% ECOG 3. Most common indications for B+R initiation were bulky symptomatic lymphadenopathy (69.1%), cytopenia (36.8%) and constitutional symptoms (36.8%). Fifty-eight percent of patients had more than one indication for therapy. Median number of B+R cycles delivered was 6 (range: 1-6), median dose of bendamustine was 90 mg/m2 (range: 45-90). Full doses of bendamustine were given in 66.7% of patients. In patients (33.3%) with dose reduction, the mean dose was 78.3 mg/m2. A total of 50.8% completed 6 cycles with no delays; and 49.2% of treatment was delayed (mean delay time :1.8 weeks). Overall response was 94.5%, with 77.1% complete and 17.4% partial remission. Median follow-up duration was 35 months (range: 4-91). At the end of follow-up, event free survival (EFS) was 77.1% and overall survival (OS) was 79.6%. Six percent of patients relapsed. In total, 14.5% develop secondary malignancies (6.5% non-hematological and 8% hematological) including 14 cases (7%) of aggressive B-cell lymphoma and 2 cases of myelodysplastic syndroms. A total of 16.9% of patients required support with G-CSF. The total number of adverse events was the highest in cycle one and decreased consistently with each cycle. Grade 3-4 neutropenia was recorded in 17.4%, febrile neutropenia in 8.0%, grade 3-4 anemia in 8.0%, and grade 3-4 thrombocytopenia in 3.9% of patients. Rituximab-associated infusion reactions were the most common in cycle 1 (46%) and 2 (16%). The rate of skin rash, fatigue, nausea/vomiting and infections were similar in each cycle. A total of 84.6% of patients proceeded to rituximab maintenance.

Conclusion
B+R chemoimmunotherapy was feasible to administer and retained its efficacy in non-clinical trial (real world) setting. HIgehr than previously reported rate of aggressive B-cell lymphoma was noted. No new adverse events of secondary non-hematological malignancies were found.
Keyword(s): Bendamustine, Mantle cell lymphoma, Non-Hodgkin's lymphoma, Rituximab


